-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2013; 3(1): 18-28
doi:10.5923/j.env.20130301.03
Distribution and Speciation of Heavy Metals in Crude Oil Contaminated Soils from Niger Delta, Nigeria
Ideriah T. J. K. 1, Ikpe F. N. 2, Nwanjoku F. N. 3
1Institute of Pollution Studies, Rivers State University of Science and Technology, P.M.B. 5080, Port Harcourt. Rivers State, Nigeria
2Department of Crop and Soil Science, Rivers State University of Science and Technology, P.M.B. 5080, Port Harcourt. Rivers State, Nigeria
3Institute of Geosciences and Space Technology, Rivers State University of Science and Technology, P.M.B. 5080, Port Harcourt. Rivers State, Nigeria
Correspondence to: Ideriah T. J. K. , Institute of Pollution Studies, Rivers State University of Science and Technology, P.M.B. 5080, Port Harcourt. Rivers State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Soil samples at depths of 0-15cm and 15-30cm were collected with an auger around an abandoned crude oil well with a history of spillage and from control sites at Abara and Ozuzu during the rainy and dry seasons. The speciation (exchangeable, carbonate, Fe-Mn oxide, organic and residual fractions) of the metals was determined using sequential extraction methods. The exchangeable fraction had the highest concentrations and the residual fraction had the lowest concentrations. In the dry season 22.27% Pb, 25.41% Cu, 37.78% Cd, 22.24% Cr, 24.84% Ni, 21.43% Mn and 22.43% Zn were bound to the exchangeable fraction. The heavy metals in the area were potentially bioavailable since the soils are acidic (pH 4.70 – 6.70), have low silt and clay contents and higher metal concentrations were associated with the non residual fractions. The soils in the area are contaminated but do not pose serious adverse effect to humans and the environment for now as the concentrations of the heavy metals were generally within or below permissible limits recommended by the Rivers State Ministry of Environment. Regular monitoring of heavy metals concentrations in the area was recommended. The re-distribution of heavy metals and other contaminants reduced their concentrations at the spilled site.
Keywords: Heavy Metals, Speciation, Soil, Spillage, Fractions, Residual, Exchangeable, Extraction, Control, Impacted, Non Impacted
Cite this paper: Ideriah T. J. K. , Ikpe F. N. , Nwanjoku F. N. , Distribution and Speciation of Heavy Metals in Crude Oil Contaminated Soils from Niger Delta, Nigeria, World Environment, Vol. 3 No. 1, 2013, pp. 18-28. doi: 10.5923/j.env.20130301.03.
Article Outline
1. Introduction
- Heavy metals are present in the environment and most of them are essential for animals and plants. They are natural constituents of rocks and sediments. Heavy metals contamination of the environment is of major concern because of their toxicity and threat to human life and the environment[1]. Heavy metal pollution can affect all facets of the environment but their effects are most long lasting in soils due to the relatively strong absorption of many metals onto humic and clay colloids in soil[2]. Speciation is the determination of the different physico-chemical forms of a pollutant, which together comprise its total concentration in a given sample or the determination of the distribution of an individual chemical element among different groups. For understanding the environmental behaviour (mobility, bioavailability,pathways) of heavy metals information about their physico-chemical form is generally required[3]. The environmental impact of heavy metal contaminants strongly depends on the metals speciation, mobility and bioavailability in soils. Contamination of soils with heavy metals poses a long term risk to ground water and ecosystem. The presence of heavy metals in soils may lead to increased uptake by plant roots, with potential toxic effects on the plants, and the animals that depend on them. Geochemical forms of heavy metals in soil affect their solubility, which directly influence their bioavailability[4]. Therefore, determining total content of heavy metals is insufficient to assess the environmental impact of contaminated soils and sediments (Salomons and Forstner, 1980[5], because it is the chemical form that determines metal behaviour in the environment and its remobilization ability. In environmental studies speciation gives more information on the nature and state of the trace metals[6]. Predicting the partitioning of trace metals between soil and soil solution requires an understanding of the many means by which a metal can be bound in the soil[7]. The total heavy metal amount in soils is distributed into five fractions(i) Soluble and exchangeable fraction; (ii) bound to carbonate phases, (iii) Bound to reducible phases (Fe and Mn oxides), (iv) Bound to organic matter and sulphides, and (v) Detritus or lattice metals[5]. Heavy metals present in these categories have different remobilization behaviours under changing environmental conditions[8]. Numerous extraction schemes for soils and sediments have been described in the literature[9, 10]. The extraction scheme used here is based on operationally defined fractions- water soluble, exchangeable, carbonate, Fe – Mn oxides, organic, and residual fractions. Recently, attempts have been made to assess the bioavailability of heavy metals in contaminated soils and sediments using sequential extraction[11]. Such assessments assume that metal bioavailability decreases with each successive extraction step in the procedure. Therefore, metals in water soluble and exchangeable fractions would be readily bioavailable to the environment; whereas the metals in the residual fractions are tightly bound and would not be expected to be released under natural conditions[11, 12].The procedure of[9] is one of the most thoroughly researched and widely used procedures to evaluate the efficacy of decontamination treatments. The incessant crude oil spillage arising from the oil production activity has impacted negatively on terrestrial and aquatic resources. The situation is increasingly becoming a concern to the people of the area as oil companies are expanding and waste treatment facilities are either inadequate or not available. Therefore this study aims to determine the speciation of selected heavy metals such as copper, lead, nickel, chromium, cadmium, manganese and zinc in the soil. The data obtained in this study will aid in the establishment of comprehensive monitoring system and information gathering on heavy metals in the environment which are essential components of any pollution control system.
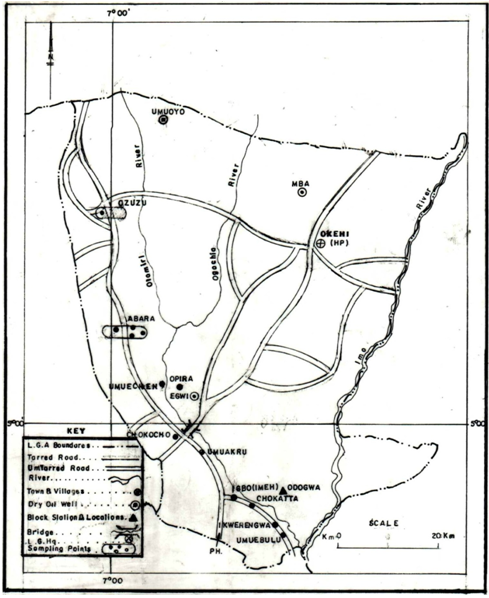 | Figure 1. Map of the study area showing sampling points |
2. Materials and Methods
2.1. Description of Study Site
- The study area is the Ola-1 oil spill site at Umuola in Abara community, in Etche local government area of Rivers State. Etche lies within latitude 5° and 6° N and longitude 4° and 5° E. Etche occupies the North – Eastern part of Rivers State. It is bounded on the North by Imo State, on the West by Ikwerre local government area and on the South – East by Imo River an extension of Imo State. (Fig.1) The area experiences the normal Niger Delta climatic conditions. The climate of the area is basically that of the equatorial tropical rainfall occurring almost through the year except in December, January and February, which are not completely free from rainfall in some years. The annual rainfall of the area is about 2, 405.2mm[13]. Annual mean air temperature is 31.3℃; the highest monthly mean temperature was 29.7℃ in (August), and the lowest monthly mean temperature is 27.5℃ in (January). There are several human activities including commercial and agricultural activities in the area.
2.2. Sample Collection, Preparation and Analyses
- Soil samples were collected with an auger during rainy (September) and dry (February) seasons at five locations (3 at spill site and 2 at control sites). Three sampling points were randomly chosen at each of the five sampling locations. Soil samples from nine (9) sampling points within the impacted area and six (6) from non impacted (control) areas were collected. A total of thirty samples comprising fifteen 0-15 cm depths and fifteen 15-30cm depths were collected into polythene bags using hand auger at each season. Ten (10) composite samples comprising of five 0-15cm and five 15-30cm were made from the thirty samples and taken to the Laboratory for preparation and analyses at each season. The composite samples were air – dried, ground to pass through a 2mm mesh and stored at room temperature in well – labelled polythene bags.
2.3. Physico-chemical Properties
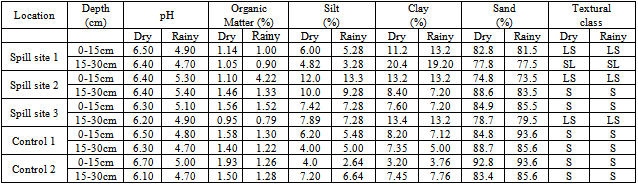
- Standard methods were used to analyse the physico-chemical parameters such as pH, silt, clay and sand[14] and organic matter[15].
2.4. Sequential Extraction of Metals
- One gram of soil was used in the sequential extraction process. The method adopted was that of[16] with slight modification.
2.5. Extraction of Exchangeable Metals
- Forty millilitres (40ml) of 1M MgCl2 were added to the residue and this was agitated for 10 hours in a mechanical shaker.
2.6. Extraction of Carbonate Bound Metals
- Forty millilitres (40ml) sodium acetate (NaOAc) was added to the residue at pH =5 with continuous agitation for 5 hours.
2.7. Extraction of Fe/Mn Oxide Bound Metals
- One hundred millilitres (100ml) of 0.04M NH2OH. HCl in 25% acetic acid were added to the residue and the mixture was heated in a thermo stated water bath to 96±2℃ for 2 hours with occasional agitation. An additional 3ml of 30% H2O2 were added and the mixture heated for 3 hours.
2.8. Extraction of Organically Bound Metals
- Three millilitres (3ml) of 0.02M HNO3 and 5ml of 30% H2O2 (pH =2.0) was added to the residue, and the mixture heated in a water bath to 85±2℃ for 2 hours with occasional agitation. An additional 3ml of 30% H2O2 was added and the mixture heated for 3 hours.
2.9. Extraction of Residual Forms of Metals
- The residue from the extraction process was washed with distilled water, and the supernatant discarded. Then 5ml HF and 10ml HClO4 were added to the residue and the sample digested. The digest were filtered and the filtrate analysed for residue bound metal ions.
3. Results and Discussion
3.1. Results
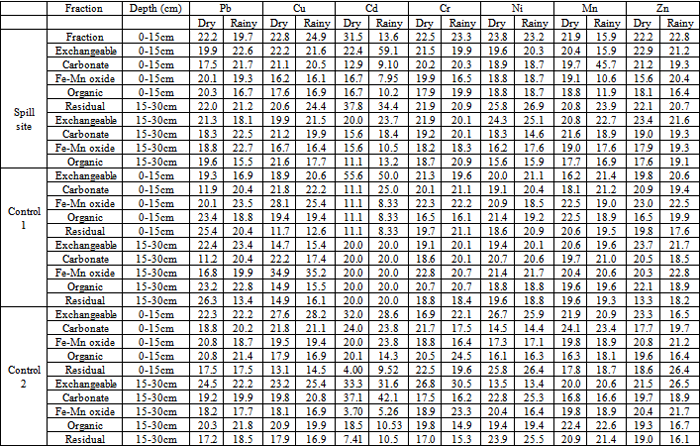
- The results of the concentrations and percent distribution of heavy metals in various fractions are presented in Tables 1 and 2 while Table 3 shows some physico-chemical parameters measured in the soil.The dry season and topsoil (0-15cm) concentrations of Pb, Cu, Ni, Mn and Zn were higher than the rainy season and subsoil (15-30cm) concentrations at the spill site. Similar variations were observed at the control sites. The reverse was observed in the concentrations of Cr. (Table 1).Table 2 shows the percentage distributions of the metals in each fraction in the dry and rainy seasons. At the spill site the highest concentration of Pb 22.2% (0-15cm) was obtained in the exchangeable fraction in the dry season and 22.7% (15-30cm) in the organic fraction in the rainy season. At the control sites the highest concentration of Pb 26.3% (15-30cm) was obtained in the Residual fraction at control 1 in the dry season and 23.5% (0-15cm) in the Fe-Mn oxide fraction at control 1 in the rainy season. The least concentration of Pb, 11.2% (15-30cm) was obtained in the carbon fraction at control 1 in the dry season and 13.5% (15-30cm) in the residual fraction at control 1 in the rainy season.
|
|
|
3.2. Discussions
- The dry season concentrations of the various forms of the heavy metals except Cr were higher than the rainy season concentrations. Similarly, the topsoil (0-15cm) concentrations of the various fractions of the heavy metals were higher than the subsoil (15-30cm) concentrations. These observations are attributed to the cleansing effect of rainfall during the rainy season and the accumulation of heavy metals at the 0-15cm depth due to automobile emissions and other human activities. The dry and rainy seasons concentrations of the metals showed significant difference (p<0.05) for all the metals except Cr (p>0.05) with correlation coefficients r = 0.6981(Pb), r = 0.9570(Cu), r = 0.0436(Cd), r = 0.8579(Cr), r = 0.7207(Ni), r = 0.5765(Mn) and r = 0.2751(Zn). Statistical analysis also showed that the difference between the dry and rainy season concentrations of the various fractions were significant (p<0.05). The trend of the heavy metals at the spill site and control sites were similar. However Cu and Cr did not show any particular trend. Cr and Ni concentrations were higher at the 15-30cm depth than the 0-15cm depth at the control sites. This observation is attributed to tiling of the soil during cultivation and abandoned metal parts buried in the soil. The concentrations of the carbonate, Fe-Mn oxide and organic matter fractions at the spill site were higher than those at the control sites. This indicates that the heavy metals were more available at the spill site than at the control sites. The difference in concentrations of the fractions between the spill site and control sites were not significant (p>0.05) except in the carbonate fraction between the spill site and control site 2 which was found to be significant (p<0.05). The fractions between the spill site and control 1 showed high positive correlations, r = 0.8856 (exchangeable), r = 0.9230 (carbonate), r = 0.7945 (Fe-Mn oxide), r = 0.9434 (organics) and r = 0.9615 (residual) and between the spill site and control 2 showed r = 0.8935 (exchangeable), r = 0.9580 (carbonate), r = 0.7269 (Fe-Mn oxide), r = 0.8146 (organic) and r = 0.8248 (residual). Analysis of variance (ANOVA) on the dry season results showed significant difference (p<0.05) between the various fractions at the spill site but no significant difference (p>0.05) at the control sites. However, in the rainy season the reverse was the case at the spill site and control 1 but similar at control 2. This is attributed to the fact that both the spill site and control 1 are cultivated areas.The differences in concentrations of the metals between the depths, 0-15cm and 15-30cm, at the spill site showed significant difference (p<0.05) with coefficient of correlations, r = 0.9973 (exchangeable) and r = 0.9978 (carbonate) but no significant difference (p>0.05) with r = 0.7692 (Fe-Mn oxide), r = 0.9976 (Organic) and r = 0.9963 (Residual). However there was no significant difference (p>0.05) in all the fractions at control 2 with coefficient of correlations, r = 0.9654, 0.9781, 0.9957, 9760 and 0.9707 for exchangeable, carbonate, Fe-Mn oxide, organic and residual fractions respectively.The concentrations of the metals were compared with permissible limits 2-20 mg kg-1 (Pb), 2-250 mg kg-1 (Cu), 0.03-3 mg kg-1 (Cd), 10-200 mg kg-1 (Cr), 5-500 mg kg-1 (Ni), 200-3000 mg kg-1 (Mn) and 50-300 mg kg-1 (Zn)[17, 18, 19]. The concentrations of Pb, Cu and Cd were within their limits while the concentrations of Cr, Ni, Mn and Zn were below their limits. This implies that the soils in the area are contaminated with metals and do not pose serious environmental and health concern for now.The highest concentration of Pb, 22.2% (0-15cm) at the spill site was obtained in the exchangeable fraction in the dry season and 22.7% (15-30cm) in the organic fraction in the rainy season (Table 2). The highest concentration of Pb 26.3%(15-30cm) was obtained in the residual fraction at control 1 in the dry season and 23.5% (0-15cm) in the Fe-Mn oxide fraction at control 1 in the rainy season. The highest concentrations of Pb were obtained at controls 1 and 2 in the dry and rainy seasons, respectively. This implies that the oil spill was not the major source of Pb in the area. Furthermore since control 1 is closer to a high traffic road, emissions from automobiles could, perhaps, be responsible for the levels of Pb in the area. The association of Pb with different fractions followed the order: 1) Spill site:-Dry season: Exchangeable > Carbonate >Residual > Organic > Fe-Mn oxideRainy: Fe-Mn oxide > Organics > Exchangeable > Carbonate > Residual 2) Control 1:- Dry season: Residual > Organic > Exchangeable > Fe-Mn oxide > Carbonate.Rainy season:- Fe Mn oxide > Organic > Carbonate > Exchangeable > Residual 3) Control 2:-Dry season: Exchangeable > Organic > Fe-Mn oxide> Carbonate > Residual.Rainy season: Exchangeable > Organic > Carbonate > Fe-Mn oxide > Residual Pb is therefore bioavailable at the spill site and control 2. At the spill site the highest concentrations of Cu, 22.8% (0-15cm) and 24.9% (0-15cm) were obtained in the exchangeable fraction in the dry and rainy seasons, respectively. At control 1 the highest concentrations of Cu, 34.9% (15-30cm) and 35.2% (15-30cm) were obtained in the Fe-Mn oxide fraction in the dry and rainy seasons respectively. The highest amount of Cu obtained at the control implies that the spill was not the major source of Cu in the area. The ranking of different forms of Cu was found to be as follows: 1) Spill site:-Exchangeable > Fe-Mn oxide > Carbonate > Residual > Organic Exchangeable > Carbonate > Fe-Mn oxide > Residual > Organic2)Control 1:-Dry season: Fe-Mn > Carbonate > Organic > Exchangeable > Residual.Rainy season: Fe-Mn oxide > Carbonate > Exchangeable >Organic > Residual 3) Control 2:- Dry season: Exchangeable > Carbonate > Organic > Fe-Mn oxide > Residual. Rainy season: Exchangeable > Carbonate > Organic > Fe-Mn oxide > ResidualThe above trends indicate that Cu was bioavailable at the study area. This observation agrees with the report[20] which stated that Cu was associated with the nonresidual fractions which increases potential Cu mobility and bioavailability in soils. At the spill site the highest concentration of Cd, 37.8% (15-30cm) occurred in the exchangeable fraction in the dry season and 59.1% (0-15cm) in the carbon fraction in the rainy season. The highest concentrations at control 1 were 55.6% (0-15cm) and 50.0% (0-15cm) in the exchangeable fraction in the dry and rainy seasons, respectively. The highest amounts of Cd at the spill site imply that the spill was the major source of Cd at spill site. The association of Cd with different fractions followed the order: 1) Spill site:-Dry season: Exchangeable > Carbonate > Fe-Mn oxide > Residual Rainy season: Carbonate > Exchangeable > Fe-Mn oxide > Residual > Organic 2) Control 1:- Dry season: Exchangeable > Carbonate = Fe-Mn oxide = Organic = ResidualRainy season: Exchangeable > Carbonate = Fe-Mn oxide = Organic = Residual 3) Control 2:- Dry season: Exchangeable > Carbonate > Organic > Fe-Mn oxide > Residual Rainy season: Carbonate > Exchangeable > Fe-Mn oxide > Organic > ResidualThese trends suggest that potentially more Cd was found in nonresidual than in the residual fractions. Thus, Cd was bioavailable at the study area because heavy metals present in the exchangeable fraction are usually thought to be readily available for plant uptake[11]. These observations agree with the reports[21, 10, 22] which observed that Cd was more in the nonresidual fraction. On the contrary, the trend of Cd was different from other reports[23, 24] which found that Cd was high in the residual fraction. This difference might be attributed to the differences in humic acid composition from different locations and also the modification of humic acid – metal interactions by other soil components. The highest concentrations of Cr, 2.52% (0-15cm) and 23.4% (0-15cm) at the spill site were obtained in the exchangeable fraction in the dry and rainy seasons, respectively. The highest concentration of Cr, 22.4% (0-15cm) at control 1 occurred in the Fe-Mn oxide fraction in the dry season and 30.4% (15-30cm) in the exchangeable fraction at control 2 in the rainy season. The highest amount of Cr obtained at the control sites, implies that the spill was not the main source of Cr in the area. 1) Spill site:-Dry season: Exchangeable > Carbonate > Fe-Mn oxide > Organic > Residual Rainy season: Exchangeable > Residual > Fe-Mn oxide > Carbonate > Organic 2) Control 1:- Dry season: Fe-Mn oxide > Exchangeable > Carbonate > Residual > OrganicRainy season: Fe-Mn oxide > Carbonate > Exchangeable > Residual > Organic 3) Control 2:-Dry season: Exchangeable > Organic > Residual > Carbonate > Fe-Mn oxideRainy season: Exchangeable > Fe-Mn oxide> Organic > Residual > CarbonateThus, Cr was bioavailable at the study area with control 2 having higher amount than control 1.The highest concentration of Ni, 25.9% (15-30cm) and 26.9% (15-30cm) at the spill site occurred in the exchangeable fraction in the dry and rainy seasons respectively. At the control sites the highest concentration of Ni 26.3% (0-15cm) and 25.9% (0-15cm) occurred in the exchangeable fraction at control 2 in the dry and rainy seasons, respectively. The highest amount of Ni was at control 2 in both seasons; implying that the spill was not the major source of Ni in the area. The association of Ni with the different fractions followed the order: 1) Spill site:-Dry season: Exchangeable > Carbonate > Fe-Mn oxide > Organic > ResidualRainy season: Exchangeable > Carbonate > Organic > Residual > Fe-Mn oxide2) Control 1:-Dry season: Residual > Fe-Mn oxide > Organic > Carbonate > Exchangeable Rainy season: Exchangeable > Carbonate > Fe-Mn oxide > Residual > Organic3) Control 2:-Dry season: Residual > Exchangeable > Fe-Mn oxide > Carbonate > Organic Rainy season: Residual > Carbonate > Exchangeable > Organic > Fe-Mn oxideThus, while Ni was not bioavailable at control 2, it was bioavailable at the spill site. The highest concentration of Mn, 21.9% (0-15cm) was obtained in the exchangeable fraction in the dry season and 45.7% (0-15cm) in the Fe-Mn oxide fraction in the rainy season. At the control sites the highest concentrations of Mn were 22.5% (0-15cm) in Fe-Mn oxide and organic fraction at control 1 in the dry season and 23.4% (0-15cm) in the carbon fraction at control 2 in the rainy season. The highest amount of Mn obtained at the spill site was likely due to the oil spill. The ranking of different forms of Mn was found to be as follows: 1) Spill site:-Dry season: Exchangeable > Fe-Mn oxide >Carbonate > Organic > Residual Wet season: Fe-Mn oxide > Exchangeable > Carbonate > Residual > Organic2) Control 1:- Dry season: Fe-Mn oxide > Organic > Residual > Carbonate >Exchangeable Rainy season: Carbonate > Exchangeable > Fe-Mn oxide > Residual > Organic. 3) Control 2:- Dry season: Exchangeable > Carbonate > Fe-Mn oxide > Residual >Organic Rainy season: Exchangeable > Organic > Residual > Carbonate > Fe-Mn oxideThus, Mn was generally bioavailable in the study area. The highest concentration of Zn at the spill site, 23.4% (15-30cm) was in the carbonate fraction in the dry season and 22.9% (0-15cm) in the exchangeable fraction in the rainy season. The highest of Zn concentration at the control 1, 23.7% (15-30cm) was in the exchangeable fraction in the dry season and 26.5% (15-30cm) in the exchangeable fraction at control 2 in the rainy season. The highest amount of Zn was obtained at the spill site, suggesting that the spill influenced the level of Zn in the area. The association of Zn with the various fractions followed the trend: 1) Spill site:- Dry season: Carbonate > Exchangeable > Fe-Mn oxide > Residual > OrganicRainy season: Exchangeable > Carbonate > Organic > Fe-Mn oxide > Residual 2) Control 1: Dry season: Exchangeable > Fe-Mn oxide > Carbonate > Organic > Residual Rainy season: Fe-Mn oxide > Exchangeable > Organic > Carbonate > Residual 3) Control 2:- Dry season: Exchangeable > Fe-Mn oxide >Organic > Residual > Carbonate Rainy season: Exchangeable > Fe-Mn oxide > Residual > Carbonate > Organic The above trend indicates that Zn was bioavailable in the area. It was observed that all the heavy metals except Pb and Cu at all the sites and at control 1 were high in the exchangeable form. This does not agree with the report[24] which stated that the non residual fraction contained the highest concentrations of Pb and Cu while Cd was highest in carbonate fraction but agrees with[20] which observed that a significant amount of Zn was associated with the nonresidual fractions in soils. This suggests that most of the heavy metals in the area especially at the spill site are potentially bioavailable because greater amounts were associated with the non residual fractions[11, 20]. Cd in the rainy season at spill site and control 2, Mn in the rainy season at the controls and Zn in the dry season at spill site, had high prevalence of carbonate fraction. This is in agreement with the reports[24, 25] which stated that high prevalence of these metals occurred in the acid soluble (carbonate) fraction. High proportions of Cu, Cr and Mn (at control 1, both seasons) and Zn (at control 1, rainy season) occurred in Fe-Mn oxide form. This agrees with the report[26] which observed that these metals occurred in Fe-Mn oxide fraction in soils amended with composts.High concentrations of Ni (at control 1, dry season and control 2 in both seasons) were found in the Residual fraction. Trace elements in the residual forms are completely immobile and not bioavailable[26]. In this study no heavy metal was predominant in the organic fraction. This is not in line with[24, 26] which found large proportion of heavy metals bound to the organic fraction. The presence of heavy metals in the residual fraction may be due to the presence of acid resistant mineral and organic materials. Owing to the predominantly sandy nature of the soils at the control sites, the heavy metals may have co-precipitated with various silicate species consequent to their adsorption into the mineral lattice[27]. Trace metals in the residual fractions remained relatively stable and inert and they are not easily released into the mobile and bioavailable phases[9]. Carbonates have been implicated as immobilizing most heavy metals by providing an adsorbing or nucleating surface and by buffering the soil pH[28]. In this study, based on the dry season results, 20.6% Pb (spill site), 22.0% Cu (control 1), 30.5% Cd (control 2), 21.7% Cr (spill site), 21.9% Ni (spill site), 20.6% Mn (spill site) and 23.2% Zn (spill site) were bound in the carbonate fraction and agrees with[16]. According to[29], sequestration of heavy metals by carbonates is an important mechanism in the mobility and availability of heavy metals in the environment. Some studies[30, 31] reported that carbonates are only stable in soils with high pH values. The moderate soil pH obtained in this study, 4.70 – 6.70 indicate that it is unlikely that carbonates are present in the area. Acid environment enhances the mobility of metals in the environment. Metals bound to carbonate are sensitive to pH changes and they are leached by lowering the pH[32]. Heavy metals interact with organic matter through various mechanisms, which affect their bioavailability. In this study, the dry season results indicate that 23.3% Pb (control 1), 19.5% Cu (control 2), 19.3% Cd (control 2), 19.9% Cr (control 2), 20.2% Ni (control 1), 21.1% Mn (control 1) and 19.4% Zn (control 2) were found bound to organic carbon.The sequence of the heavy metals in this fraction was in the order: Pb > Ni > Mn > Cr > Cu > Zn > Cd. Iron and manganese oxides (reducible fractions) have been implicated in the sequestration of heavy metals in the environment, existing in nodules, concretions, cement between particles or as acting on particles and are excellent trace element scavengers[33]. In this study the dry season results showed that 19.6% Pb (control 2), 31.5% Cu (control 1), 15.6% Cd (control 1), 22.6% Cr (control 1), 21.2% Ni (control 1), 21.5% Mn (control 1) and 21.7% Zn (control 1) were bound in this fraction. Copper had the highest amount in this fraction. This observation is in agreement with the report[24] that high concentrations of Cu exist in the Fe-Mn oxide fraction. The fact that iron (ii) and iron (iii) precipitate as hydroxides at pH > 2.0 while manganese (ii) precipitates at a pH > 8.6 confirms that iron rather than manganese species may be the main species responsible for the sequestration of the heavy metals at the prevailing soil pH (4.7 – 6.7 in both seasons) in this study. Iron (ii) and (iii) species have been indicated to scavenge heavy metals from soil solutions that would normally not precipitate considering both thermodynamic and redox factors[34, 35]. Heavy metals retained in this form may be a long-term source of contamination since they could be released if there are changes in the redox status of the soil[36].The exchangeable fraction in the dry season showed that 22.3% Pb (control 2), 25.4% Cu (control 2), 37.8% Cd (control 1), 22.2% Cr (spill site), 24.8% Ni (spill site), 21.4% Mn (spill site) and 22.4% Zn (control 2) were bound to this fraction. Cadmium was the highest in this fraction. This is in agreement with the report[24] that Cd was the highest in the exchangeable fraction. This fraction is important because of the high mobility of heavy metals from this fraction to the aqueous phase. The potentially available (bioavailable) heavy metals comprise those that can be accessed by man through ingestion and are usually considered as being of anthropogenic origin. They may be approximately related to heavy metals in the exchangeable, and carbonate fractions. Heavy metals in the non-residual fractions, including the exchangeable, carbonate, Fe-Mn oxides and organic fractions may reflect various degrees of reactivity and potential bioavailability. They may also change depending upon the surrounding physical and geochemical conditions[9, 37, 38].
4. Conclusions
- The screening of soils around an abandoned oil well with the history of oil spillage indicated the presence and distribution of heavy metals in the area. Since the levels of heavy metals studied were generally low and were either within or below natural and permissible limits found in unpolluted soils, the soils in the study area though considered to be contaminated do not pose any adverse effect to humans and the environment. The soils under study showed spatial variation in the concentrations of heavy metals in the area. The spatial variations were due to differences in moisture content, silt and clay contents, organic matter content and pH of the different sites.The seasonal variations in the concentrations of the heavy metals resulted from differences in individual heavy metal, pH, topography of the area and leaching by acid rain during the rainy season. Knowledge of heavy metal composition of soils is not adequate to determine heavy metal pollution of the environment since different forms of the heavy metals have different mobility, bioavailability and potential environmental contamination. The heavy metal speciation analysis done in this work provided information on their bioavailability and mobility. The heavy metals investigated were potentially bioavailable since the soils are acidic and have low silt and clay contents. A significant amount of the heavy metals were associated with the non residual fraction in the soils studied. Therefore the heavy metals concentrations in the area should be monitored regularly. The lack of uniformity (variations) in sequential extraction methods (reagent types, strength, volume and reaction time,) made it difficult to compare the results obtained with those of other studies. Therefore, the disagreements of the results obtained in this study with other reports were not unexpected. Since heavy metal levels of soils from the control sites were some times higher than those from the spilled site, it seems that spillage was not the major source of heavy metals at the spilled site. However, the long period between the spillage and the time of study may have warranted the re-distribution of heavy metals beyond the spilled site.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML