-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2013; 3(1): 1-8
doi:10.5923/j.env.20130301.01
Ecological Studies on Macrobenthic Invertebrates of Bardawil Wetland, Egypt
Magdy T. Khalil 1, Abd El-Halim A. Saad 1, Mohamed R. Fishar 2, Tadros Z. Bedir 1
1Zoology Dept., Faculty of Science, Ain Shams University, Egypt
2National Institute of Oceanography & Fisheries, Egypt
Correspondence to: Magdy T. Khalil , Zoology Dept., Faculty of Science, Ain Shams University, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Species composition, distribution of macrobenthic invertebrates in Baradwil Wetland were studied during 2006-2007. Seasonal samples were collected from 12 stations representing different habitats of the wetland. The results revealed that Macrobenthic community is consist of 51 species belonging to phyla; Coelentrata, Nemertina Annelida, Arthropoda, Mollusca, and Echinodermata. The abundance of benthic invertebrate species was closely correlated with nature of bottom sediments, organic matter, salinity and anthropogenic activities (intensive fish trawling and artificial inlets). The standing crop of macrobenthos decreased during the last two decades due to changes in fish community structure. In the present study a remarkable increase in the population density of Mollusca was noticed because of the declining of the fish, Sparus aurata which is mainly a bottom feeder depends on molluscan animals in its diets. This change in fish community, which consequently changes the whole ecosystem of the lake, encourages us to recommend establishing a monitoring program to follow up changes in the lake ecosystem, especially the benthic fauna that will be of great help in the management of such important water body
Keywords: Bardawil Wetland, Macrobenthos, Invertebrates, Biodiversity, Ecology, Egypt
Cite this paper: Magdy T. Khalil , Abd El-Halim A. Saad , Mohamed R. Fishar , Tadros Z. Bedir , Ecological Studies on Macrobenthic Invertebrates of Bardawil Wetland, Egypt, World Environment, Vol. 3 No. 1, 2013, pp. 1-8. doi: 10.5923/j.env.20130301.01.
Article Outline
1. Introduction
- Bardawil Wetland is one of the five northern lakes in Egypt. It is bordered from the north by a convex sand barrier that separates it from the Sinai Mediterranean coast and from the south by the sand dune belt. The extreme eastern part of this lake (i.e. Zaranik Lagoon) is a Ramsar site and has been declared as a natural protectorate in 1985. This lake has an elliptical shape representing a major morphological feature along North Sinai coast. Its area is about 164,000 Feddan and extends for a distance of 80 km along N-S axis. Its maximum width is 20 km and maximum depth is 3 m. The main water body of this lake lies towards the east with an area of 58,000 Feddan of which Zaranik Lagoon occupies about 10,000 Feddan. Sea water enters the lagoon through three inlets: two artificial tidal inlets and a natural eastern inlet of Zaranik which is now occasionally closed by silting. Lake Bardawil had been the subject of many studies during the last 20 years. Many theses, reports and papers had been published covering the geomorphology, morphometry, sedimentology, hydrology and water quality, macrophytes and phytoplankton, zooplankton and zoobenthos, fishes and fisheries, avifauna and others[1-3].On the other hand, the benthic invertebrates in aquatic ecosystems, play an important role in the transformation of the organic matter sediment on the bottom to its base elements and subsequently contribute to the basic nutrition of fish. The composition of the benthic fauna has largely been considered as a good indicator of water quality because, unlike planktonic species, they form relatively stable communities in the sediments which do not change over long time intervals and reflect characteristics of both sediments and upper water layer[4]. Benthic invertebrates in aquatic ecosystems are divided according to the size, into macrobenthos and meiobenthos. Macrobenthic invertebrates in Lakes are frequently used to evaluate the overall ecosystem “health”[5-8], because these communities are important to material cycling and secondary production, and are sensitive to environmental contaminants. To fully understand the reasons of disturbances that affect benthic community and distribution, it is important to measure the environmental factors that provide the basic ecological template structuring the benthic community.The available data dealing with the macrobenthos in lagoon Bardawil are scarce.[9] included macrobenthos in their study on ecology of Bardawil lagoon. They listed 30 species of benthic fauna and flora of the lagoon.[2] mentioned that the benthic community consists mainly of members of Annelida, Arthropoda and Mollusca.[4] studied the community structure, biodiversity, biomass and abundance of macrobenthos in the lake in relation to changes in some abiotic and biotic variables.[10] studied the community structure and biochemical parameters of annelid worms in the lake. They showed that biochemical parameters were higher in all studied specimens, during March owing to maturation of gametes and reproductive activities.[11]studied the long-term changes of Arthropoda and Mollusca. They observed dramatic changes in their assemblages.[12] carried out the latest study on macrobenthos of the lake. He surveyed a monthly sampling of macroinvertebrates from 12 sites during 2004. The objective of this work is to study species composition, biodiversity, and abundance of macrobenthic fauna community in relation to changes in some abiotic and biotic variables. As well as, following up long-term changes of this community in relation to some environmental changes.
2. Material and Methods
- Study areaLake Bardawil is a shallow, hypersaline lagoon (salinity 50.9 ‰), situated at the north of Sinai and occupying much of the Mediterranean coast of Sinai. Its coordinates are 310 09\ to 310 03\ N latitude and 330 19.25\ to 320 46.75\ E longitudes. It extends for about 80 km with a maximum width 20 km and a maximum depth of 3 meters. It is separated from the sea by sand bar that varies in width between 100 meters and 1 km (Figure 1). The lake shore is mainly bare sand, with scattered salt marsh and mudflats.Sampling Programme and stationsWater and macrobenthic samples were seasonally collected from spring 2006 to winter 2007 from 12 stations which have been selected to represent different habitats of the Lake Bardawil (Table 1 and Figure 1).MacrobenthosSeasonal macrobenthic samples were collected using Ekman Grab (225cm2 opening area). The collected samples were stored in plastic jars after adding 7 % formaldehyde solution. The samples were washed in the laboratory with tap water through a small hand net of 500 µm mesh diameter. Sorting of specimens were carried out by taking successive small subsamples under stereoscopic microscope at 40 X. The bottom macrobenthic organisms were sorted into main groups, genera or species.Physical and chemical variablesTransparency was measured by Secchi disk (SD) (25 cm in diameter), according to[13]. Water temperature and dissolved oxygen were measured at the sampling sites during the day time, using an oxygen meter (model YSI 58) equipped with a temperature sensor. The pH values were measured using glass electrode pH meter (digital minicomputer pH-meter model Carrning 354). Nitrogen compounds (nitrate-nitrogen) were measured by phenol-disulphonic acid method according to[14]. Spectrophotometer used was of model Milton Roy 21D. Dissolved orthophosphate was measured according to[15], using Spectrophotometer (model Milton Roy 21D).
|
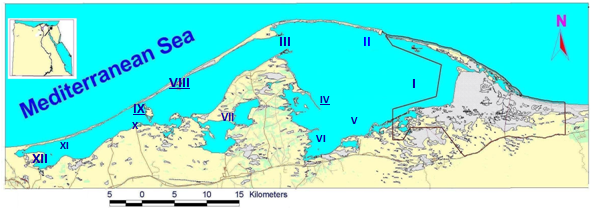 | Figure 1. A Map Showing the Location of Bardawil Lagoon and the Selected Stations |
3. Results and Discussion
2.1. Physical and Chemical Properties of Water
2.1.1. Water Temperature
- The recorded values of water temperature at the surveyed stations fluctuated between 28.3 ℃ in the summer to 18.9 ℃ in winter and dropped to a minimum of 17.3 ℃ at station II (Boughaz II) in the same seasons and gradually increased until reached maximum of 33.9 ℃ at station VII in spring season. The same results obtained by[3].
2.1.2. Transparency
- Turbidity of lake water is caused by mineral or organisms particles in suspension. Mineral turbidity is composed of clay and silt particles, while organic turbidity is made up of planktonic organisms. Lake Bardawil water shows high visibility values that fluctuated between 121 cm in winter and 175 cm in spring. An average annual maximum and minimum values of Secchi disc readings reached 227.5 cm at station IX and 89cm at station III respectively. The highest turbidity, which was observed in winter, is mainly due to continuous mixing of the lagoon water by the strong wind action which prevails in this seasons and the phytoplankton blooming in autumn[16]. On the contrary, the highest transparency found in summer is mainly due to the stability of the lagoon water, which resulted from the decrease of wind speed[16]. The same results were recorded during 2006/2007 season.
2.1.3. Total Dissolved Solids
- Total dissolved solids (TDS) in Bardawil lagoon fluctuated within a high range of 49.8 – 37.1 g/l, with an average value of 44.2 g/l. The regional average values showed high differences at all regions of the lagoon; maximum and minimum values; 67.0 and 33.5 g/l were recorded at stations I during summer 2006 and station III during autumn 2006 respectively. The seasonal average of total dissolved solids showed a maximum value in summer 2006 of 48.3 g/l to a minimum value in winter 2007 of about 41.5 g/l.During 1970's[17] pointed that, total dissolved solids exceeded 100 g/l due to the two artificial inlets were completely closed. During 1980's the value gradually decreased due to re-opening of the two inlets after Liberation of Sinai; it reached to about 90 g/l[16]. During 1990's[1] and[18] showed that the total dissolved solids values fluctuated between 40.0 - 81.5 g/l and 44 – 74 g/l respectively.
2.1.4. Salinity
- The salinity of Bardawil Lagoon is much higher than in the open sea as a result of low rainfall (80 – 100 mm/year) and high evaporation rate (1460 mm/year). Therefore, the calculated net water loss by[19] is about 2.2 million m3 per day compensated by the inward flows through the inlets. The climatic conditions play a major role in influencing the salinity of lake. The salinity of lagoon shows relative variation with the distance from the two artificial openings with the Mediterranean Sea. Moreover, it shows pronounced annual changes, with the lowest salinities during winter, and highest one during summer. The same author found that the level of the salinity in the lagoon is the state of the openings. However, in the period of partial closure of the openings by sand, the salinities are higher than after dredging. These inlets have natural tendency to be closed by sand carried along the coast by waves and eastward coastal current at a rate of 300,000 to 500,000m3 per year. The tidal flow through the two inlets is too weak to keep them open, so continuous dredging is needed during the year to remove accumulated sand by the General Authority for Fish Resources Development (Ministry of Agriculture).During 1969 till 1971 the two openings were completely blocked by the accumulated sand. Consequently, the salinities of the Lagoon rose considerably reaching to about 100 ‰ and in some isolated basin the salinity reached up to 170 ‰ ([17] and[20]). During 1990's the salinity values decreased gradually after dredging and re-opening of the two inlets, it reached to about 78 ‰[18]. The salinity values fluctuated between 54 to 68 ‰ during 2000[21]. During 2004, salinity varied between minimum values of 38.5 ‰ during winter at Boughaz II area to maximum of 74.5 ‰ during summer at the most western part of the Lagoon[22].
2.1.5. Hydrogen Ion Concentration (pH)
- The hydrogen ion concentration (pH) in the studied area were always on the alkaline side and fluctuated within a narrow range of about 7.6 – 8.3 with an average value of 8.1. The regional average pH values showed high differences at all regions in the lagoon. A minimum and maximum values of about 7.1 and 8.8 were recorded at stations I during summer 2006 and station V during winter 2007 respectively.The recorded pH levels were in agreement with those obtained by[16], who recorded values that ranged between 7.5 – 8.76 with an annual average of 8.16. It is notable that, pH values showed slight variation among different stations and months. However, pH values increased slightly during spring which was attributed to photosynthetic activity, which reduced the CO2 amount in water[23]. While the decrease of pH values during summer and early autumn may be attributed to bacterial fermentation and decomposition of organic matter[24].
2.1.6. Dissolved Oxygen
- Dissolved oxygen is one of the ecological factors key of life. Moreover, dissolved oxygen is much more important to aquatic than to terrestrial life as oxygen has a low solubility in water and is often a limiting factor for life in water. Also, oxygen is needed for all oxidation, nitrification and decomposition processes and it is controlled by some factors: respiration, photosynthesis and exchange at the air water interface. On the other hand, oxygen is the most fundamental parameter of lakes, aside from water itself. The regional average values of DO fluctuated within a wide range of about 5.3 – 6.6 mg/l with an average of 5.8 mg/l. The minimum value of DO was recorded at station I during summer 2006, and the maximum value 10 mg/l was recorded at station II during winter 2007. The low average value of DO was recorded during summer 2007 (2.7 mg/l), while the highest value was recorded during winter 2007 (7.9 mg/l). The same values were recorded by[10].[25] stated that water of Bardawil Lagoon is well oxygenated during different time intervals. The minimum DO value of 4.8 mg/l was recorded at station VI during July while the maximum of 10.2 mg/l was recorded at station I during January, with annual mean value of 7.3 ± 0.9. Dissolved oxygen values showed relative decrease during hot months which was mainly attributed to elevation of temperature that led to decrease the solubility of oxygen gas[26], in addition to water salinity increase during summer affect adversely on the solubility of oxygen gas[27]. On the other hand, the increase of DO during cold months mainly due to decreasing of temperature, prevailing winds action which permits to increase the solubility of atmospheric oxygen gas[28].
2.1.7. Nutrient Salts
- Nutrient salts are dissolved mineral salts, include phosphorus (P), nitrogen (N) and silica (Si). The nitrogenous salts are nitrates (NO3), nitrites (NO2) and ammonia (NH4). Nitrite (NO2-N)Nitrites depleted completely in some stations B. Logo. The annual average value of nitrite was 4.5 ± 3.3 µg/l, while the highest value of nitrite (19.2 µg/l) was recorded at station I (El-Telul region) during winter due to pollution point at this station represented in tailings of fishermen. The biological reduction of nitrite in the lagoon due to the uptake of nitrite into cellular amino acids by the photosynthesis of plankton, and by the action of transaminase enzyme, which decrease the nitrite, values in the studied area.[16] reported that nitrites were completely depleted during his study with some exception during winter, spring and autumn in some stations.Nitrate (NO3-N)The minimum value of nitrate 13 µg/l was recorded during November at station XII while the maximum value of 89 µg/l was recorded during January at Boughaz I area, with annual average of 42 ± 15 µg/l.[22] stated that the nitrate in Bardawil Lagoon showed that the nitrate concentration increased during spring and summer which was mainly attributed to the oxidation of ammonia yielding nitrate especially in abundant oxygen and phytoplankton during spring. Furthermore, the annual average values showed a noticeable increase of nitrate content westward at stations XI and XII which may be due to dense abundance of sea grasses in these areas, besides an expected increase at El-Telul area as a consequence of fishermen tailings in this region.Ammonia (NH4+-N)The maximum ammonia value of 138 µg/l was recorded at station I (El-Telul area) during September while the minimum value of 9 µg/l was recorded at station XI during February, with annual mean value of 48 ± 11 µg/l. Ammonia contents in Bardawil Lagoon showed homogeneous distribution with narrow horizontal fluctuations except station I which suffers from different pollution aspects. The regional average values showed a notable increase of ammonia concentration during summer and early autumn which was controlled by two important processes; ammonification and denitirfication. The two processes are not only temperature dependent but are also dependent largely on available organic substrate. Orthophosphate (PO4-3-P)The maximum orthophosphate value of 90 µg/l was recorded at station IX during April while the minimum value of 10 µg/l was recorded at station III during December, with an annual mean value of 35 ± 12.4 µg/l. The orthophosphate contents in Bardawil Lagoon showed relative increases during spring and summer more than in other seasons. This phenomenon attributed to decomposition of organic matter, leads to liberating phosphorus as one of the breakdown products, and the mobilization of phosphorus from sediments under microbial activities, especially in the presence of dissolved oxygen since, it plays an important role in controlling the rate of phosphate released from sediments to the photic zone[29] .The relative decrease of orthophosphate content during autumn, and winter attributed to its sorption on to the humic matter forming humic – iron – phosphate complexes (DHM – Fe – PO4); this reduces the total amount of available phosphate.The regional average values showed that, Boughazes (inlets) areas have the higher orthophosphate contents, it decreased gradually southward. This observation indicated that orthophosphates were transported from see water into the lagoon and consequently precipitated due to higher salinity of lagoon water than the sea water. Phosphorus forms chemically tight compounds with major cations enriched in the lagoon, these tight forms especially with magnesium (in the form of MgPO4; MgHPO4 and MgH2PO4) and calcium (in the form of CaPO4; CaHPO4 and CaH2PO4). Besides lesser values of free not-bound HPO4 and H2PO4 radicals which depend on the pH variations[30].
2.2. Macrobenthos
2.2.1. Community Composition
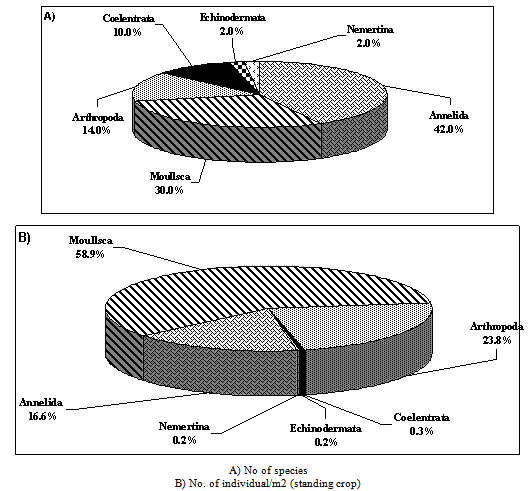 | Figure 2. Community composition of total benthos |
2.2.2. Seasonal Variation
- Concerning seasonal variation of macrobnethos, the highest population density was recorded during spring with an average of (6617 Org/m2); this is mainly attributed to the abundance and flourishment of food supply with increasing temperature. A remarkable decrease was recorded during the next season to reach (3554 Org/m2) during summer. The population density re-increased again to form the second peak with an average value of (5653 Org/m2) during spring..For the fluctuation of biomass, the highest value was recorded during spring, with an average value of (625 g/m2) and the lowest value was recorded during winter, with an average value of (197 GFW/m2).
2.2.3. Distribution
- The annual average population density of total macrobenthos was (5327 Org/m2). This value is higher than that recorded by[12] in the lake and lower than that recorded in Lake Manzalah[31] and Lake Wadi Elrayan[32].The highest population density was recorded at stations XII, VII and I with an average value of 10665, 9003 and 6453 Org/m2 respectively. On the other hand, the lowest average values were recorded at stations III, II with average values of 1093, 1049 Org/m2 respectively.Correlation between total macrobenthos and abiotic variables in lake Bardawil, temperature and total dissolved salts showed a positive correlation with standing crop of total macrobenthos (r = 0.61 and 0.86 respectively) while dissolved oxygen was negatively correlate with total macrobenthos (r = - 0.64).
2.2.4. Long Term Changes of Macrobenthos
- Five sampling programs reflecting long-term changes of macrozoobenthos in Lake Bardawil were carried out during 1984[9], 1986 -1987[33], 2003[4], 2004[12] and 2006 / 2007 (present study).Density fluctuationAs shown in Figure 3, the standing crop of total benthos during 1984 was 4164 organisms/m2. P.D. showed a remarkable decrease (3711 Org/m2) during survey of 1986 – 1987 and continued their decrease in 2003 (2230 Org/m2) followed by a sharp drop during 2004 (359 Org/m2). In the present survey (2006/2007) there was a sharp increase with an average of 5327 Org/m2
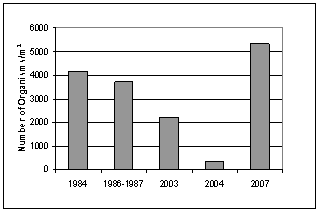 | Figure 3. Annual fluctuation of macrobenthos density during five periods (1984- 2007) |
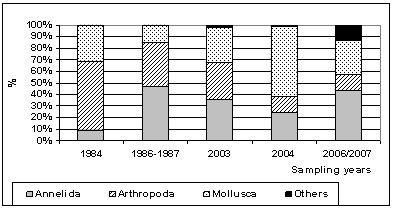 | Figure 4. Annual fluctuation of percentage composition of macrobenthic groups for different surveyed years |
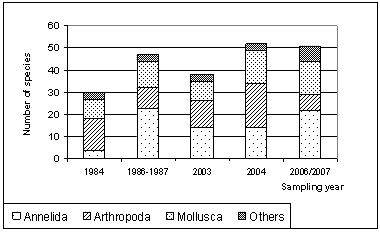 | Figure 5. Annual fluctuation of macrobenthos diversity during different surveyed years |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML