-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2012; 2(6): 116-119
doi: 10.5923/j.env.20120206.02
Physicochemical Analysis of Groundwater Samples of Bichi Local Government Area of Kano State of Nigeria
Emmanuel Bernard 1, Nurudeen Ayeni 2
1Department of Chemistry, Federal College of Education (Technical) Bichi, P.M.B 3473 Kano, Nigeria
2Department of Chemistry, Federal College of Education, P.M.B 3045, Kano, Nigeria
Correspondence to: Emmanuel Bernard , Department of Chemistry, Federal College of Education (Technical) Bichi, P.M.B 3473 Kano, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
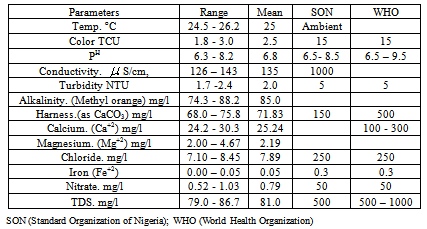
The physicochemical properties of groundwater from various locations in Bichi Local Government Area of Kano State were analysed using standard methods. The samples taken from twenty different locations revealed that the study area has a mean of Turbidity 2.0 NTU, Colour 2.5 TCU, Temp. 25℃, PH 6.8, Total Alkalinity 85.0 mg/l, Total Hardness 71.83 mg/l, and others are: Calcium 25.24 mg/l, Magnesium 2.19mg/l, Iron 0.05, Chloride 7.89mg/l, Nitrate 0.79 mg/l, Total dissolved solid 81.0 mg/l, and Conductivity 135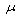 S/cm,. The study was geared towards ascertaining the quality of ground water in the area and, it was observed that the water samples were within World Health Organization (WHO) and Standard Organization of Nigeria (SON) permissible limit for ground water which satisfy the safety limit for its use for various purposes like domestic, agricultural, and industrial. It was suggested that there should be regular monitoring and control of human activities to protect the ground water from contaminations.
S/cm,. The study was geared towards ascertaining the quality of ground water in the area and, it was observed that the water samples were within World Health Organization (WHO) and Standard Organization of Nigeria (SON) permissible limit for ground water which satisfy the safety limit for its use for various purposes like domestic, agricultural, and industrial. It was suggested that there should be regular monitoring and control of human activities to protect the ground water from contaminations.
Keywords: Physicochemical, Properties, Groundwater, Bichi, Drinking, Water
Cite this paper: Emmanuel Bernard , Nurudeen Ayeni , "Physicochemical Analysis of Groundwater Samples of Bichi Local Government Area of Kano State of Nigeria", World Environment, Vol. 2 No. 6, 2012, pp. 116-119. doi: 10.5923/j.env.20120206.02.
Article Outline
1. Introduction
- Water as a universal solvent has the ability to dissolve many substances be it organic or inorganic compound. With this outstanding property, nevertheless it is almost impossible to have water in its pure form since it cannot be held up in a vacuum. Water which occurs below the water table is referred to as groundwater, it supports; drinking water supply, livestock needs, irrigation, industrial and many other commercial activities. The quality of ground water depends on various chemical constituents and their concentration, which are mostly derived from the geological data of the particular region. Groundwater is generally less susceptible to contamination and pollution when compared to surface water bodies.[1, 3]In Bichi, groundwater is one of the main sources of water used intensively for domestic and agricultural purposes, Uncensored human activities in developing countries including Nigeria contribute immensely to the poor quality of groundwater. The problems of water quality are much more acute in areas which are densely populated, with localization of industries. Importantly, groundwater can also be contaminated by naturally occurring sources. A number of chemical contaminants have been shown to cause adverse health effects in humans as a consequence of prolonged exposure through drinking-water from various sources Much of ill health which affects humanity, especially in the developing countries can be traced to lack of safe and wholesome water supply.[4-7]Water for human consumption must be free from living and non-living organisms, toxic elements and chemical substances in concentration large enough to affect health.[1, 8, 9] .The addition of various kinds of pollutants through sewage, industrial effluents, agricultural run off etc, into the water main stream brings about series of changes in the physicochemical characteristics of the water, which have been the subject of several investigations.[10, 1, 12]. In Northwest Nigeria the pollution of groundwater was traced to shallow water table that intercepts pit latrines and soaks away pits.[13]. The water used for drinking purpose should be free from toxic elements, living and non-living organisms and excessive amount of minerals that may be harmful to health. Pollution caused by fertilizers and pesticides used in Agriculture, often dispersed over large areas, is a great threat to fresh groundwater ecosystems. The water supply for human consumption in Bichi is often directly sourced from ground water without any chemical treatment and the fear of pollution has become a cause for major concern. The objective of this study is to evaluate some of the parameters that can cause contamination and in what concentration if present, and comparing it with set standards of World Health Organization and Standard Organization of Nigeria.
2. Materials and Methods
2.1. Description of Study Area
- Bichi Local Government Area is hosted by Kano state which is ranked second in population with about 9.0million people and lies between latitude 11°30′ and 11.5°N and longitude 8°30′ and 8.5°E. Nigeria is located approximately between latitude 4o and 14o North of the equator, and between longitude 2° 2’ and 15° east of the Greenwich meridian.[15]
2.2. Collection/Treatment of Water Samples
- Samples were collected from twenty locations of drinking water in plastic containers previously washed with detergents and HNO3 acid and later rinsed with sampled water several times. 2M HNO3 was added to samples for metallic ions determination, this is to maintain the stability of the oxidation state of the various elements in solution and prevent precipitation.[15][16][17]
2.3. Physicochemical Analysis
- TemperatureThe temperatures of the samples were measured at the point of collection using mercury in glass thermometer.Electrical conductivity and PH The conductivity of the samples was determined using a Jenway model 4010, and the PH Meter, model PBS – 51, EL – Hama instrument was used to determine the PH value. TurbidityA turbidimeter model HACH 2100Q Colorado, was used to determine the turbidity of the samples. Total AlkalinityIt was determined by titrimetric method using standard solution of 0.01M HCl and methyl orange as indicator.Total HardnessIt was measured using EDTA (Ethylene – Di amine Tetra – Acetic Acid) as titrant with ammonium chloride and ammonium hydroxide buffer solution and Erichrome Black T as indicator.Chloride contentIt was determined by Mohr’s method using silver nitrate as titrant and potassium chromate solution as indicator.Total Dissolved SolidThis was determined by evaporation method in an oven maintained at 200oC for 2hrs.Calcium HardnessCalcium hardness was determined using EDTA method with murexide (ammonium purpate) as indicator.The Cationics 100cm3 of the water sample was pre-concentration by heating in a vacuum until the sample was reduced to 25cm3. Determination was done using the atomic absorption spectrophotometer, Model Alpha 4 for magnesium and iron and flame photometer, model PF7, Jenway for calcium
3. Results and Discussion
- The result of physical and chemical parameters obtained from the analysis of water samples are shown in Table 1.The value of temperature in the study area ranged from 24.5 - 26.2℃. It is noted that high water temperature enhances the growth of micro organisms and may increase taste, odour, colour and corrosion problems.The colour ranged from 1.8 - 3.0 TCU which is within WHO and SON permissible limit. Colour in drinking-water is usually due to the presence of colour organic matter (primarily humic and fulvic acids) associated with the humus fraction of soil or the presence of iron and other metals, either as natural impurities or as corrosion products.[14] The PH values of samples range is 6.3 - 8.2 which conform to WHO and SON standard for drinking water. Although pH usually has no direct impact on consumers, it is one of the most important operational water quality parameters.The conductivity concentrations range were 126 – 143
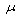 S/cm which were below SON standard for drinking water.The turbidity value of the study area ranged between 1.7 – 2.4. Turbidity in drinking-water may be due to the presence of inorganic particulate matter in some groundwater or sloughing of biofilm within the distribution system. High turbidity value can protect micro organisms from the effects of disinfection thereby can stimulate bacterial growth. The value is within WHO and SON standard for drinking water.[16][17]The alkalinity of water may be caused by dissolved strong bases such as sodium or potassium hydroxide (and other hydroxide containing compounds), hydroxide ions are always present in water, even if the concentration is extremely small. The alkalinity value ranged between 74.3 - 88.2 mg/l. When water has high alkalinity it is concluded that it is well buffered. It resists a decrease in pH when acidic rain snowmelt, enters it. If water has an alkalinity below about 100mg/L as CaCO3, it is poorly buffered and pH sensitive. This could be harmful to the plants and animals that live there.[8][14][16] The range of hardness analysed is 68.0 - 73.8 mg/L and fell below W.H.O and S.O.N standard of drinking water. Hardness caused by calcium and magnesium usually results in excessive soap consumption and subsequent “scum” formation. In some instances, consumers tolerate water hardness in excess of 500 mg/l. Depending on the interaction of other factors, such as pH and alkalinity, water with hardness above approximately 200 mg/l may cause scale deposition in the treatment works, distribution system and pipe work and tanks within buildings. Soft water, with a hardness of less than 100 mg/l, may, have a low buffering capacity and so be more corrosive for water pipes.[16][17][18]
S/cm which were below SON standard for drinking water.The turbidity value of the study area ranged between 1.7 – 2.4. Turbidity in drinking-water may be due to the presence of inorganic particulate matter in some groundwater or sloughing of biofilm within the distribution system. High turbidity value can protect micro organisms from the effects of disinfection thereby can stimulate bacterial growth. The value is within WHO and SON standard for drinking water.[16][17]The alkalinity of water may be caused by dissolved strong bases such as sodium or potassium hydroxide (and other hydroxide containing compounds), hydroxide ions are always present in water, even if the concentration is extremely small. The alkalinity value ranged between 74.3 - 88.2 mg/l. When water has high alkalinity it is concluded that it is well buffered. It resists a decrease in pH when acidic rain snowmelt, enters it. If water has an alkalinity below about 100mg/L as CaCO3, it is poorly buffered and pH sensitive. This could be harmful to the plants and animals that live there.[8][14][16] The range of hardness analysed is 68.0 - 73.8 mg/L and fell below W.H.O and S.O.N standard of drinking water. Hardness caused by calcium and magnesium usually results in excessive soap consumption and subsequent “scum” formation. In some instances, consumers tolerate water hardness in excess of 500 mg/l. Depending on the interaction of other factors, such as pH and alkalinity, water with hardness above approximately 200 mg/l may cause scale deposition in the treatment works, distribution system and pipe work and tanks within buildings. Soft water, with a hardness of less than 100 mg/l, may, have a low buffering capacity and so be more corrosive for water pipes.[16][17][18]
|
4. Conclusions
- Informing the public of the state of their drinking water should be considered as an important aspect of social responsibility as well as scientific research. Consumers may be aware of a potential problem with the safety of drinking water because of media coverage or access to research work. Lack of confidence in the drinking-water or the authorities may drive consumers to alternative, potentially less safe sources.[3][10] Not only do consumers have a right to information on the safety of their drinking-water, but they have an important role to play in assisting the authorities by their own actions and by carrying out the necessary measures at the individual level. Safe drinking water is vital to sustain life and a satisfactory (adequate, safe and accessible) supply must be available to all. Improving access to safe drinking-water can result in tangible benefits to health. Every effort should be made to achieve drinking-water quality as safe as practicable.[1][5]The results obtained from the analysis of the samples revealed that the quality of ground water in Bichi been assessed by comparing each concentration with the standard desirable limit for drinking water as prescribed by WHO and SON, are within permissible limits.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML