-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2012; 2(6): 110-115
doi: 10.5923/j.env.20120206.01
Microbial Treatment of Lateritic Ni-ore for Iron Beneficiation and Their Characterization
N. Pradhan 1, R. R. Nayak 2, D. K. Mishra 1, E. Priyadarshini 1, L. B. Sukla 1, B. K. Mishra 1
1Institute of Minerals and Materials Technology, Council of Scientific & Industrial Research (CSIR), Bhubaneswar, 751013, India
2Indian Institute of Chemical Technology, Council of Scientific & Industrial Research (CSIR), Hyderabad, 500 007, India
Correspondence to: L. B. Sukla , Institute of Minerals and Materials Technology, Council of Scientific & Industrial Research (CSIR), Bhubaneswar, 751013, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study aims at studying effect of anaerobic dissimilatory iron (III) reducing bacterial consortium on different phases of iron present in lateritic nickel ore. Such conversion in lateritic nickel ore are helpful in better recovery of sorbed metal values like Ni and Co by subsequent bioleaching or acid leaching. Here properties of thermally and microbially reduced lateritic nickel ore are compared vis-à-vis original ore. An anaerobic dissimilatory iron (III) reducing bacterial consortium capable of using glucose as carbon source and lateritic nickel ore as terminal electron acceptor was used for microbial reduction of ore under anaerobic condition. Microbial reduction changes the initial light brown colour of the lateritic nickel ore to dark brown. The change in colour is due to the conversion of goethite to magnetite, which is confirmed from the XRD pattern. The FTIR spectra and the UV-Visible spectra support the presence both goethite and hematite. The study shows that changes in phases brought about by microbial treatment are different than those by thermal treatment. The carrier mediated exchange interaction between Fe2+ and Fe3+ ions in lateritic nickel ore sample treated with IRB consortium is responsible for higher ferromagnetic ordering. The thermal reduction of the same sample showed lowering of ferromagnetic ordering due to the decreasing percentage of Fe2+ and Fe3+ ions.
Keywords: Iron Reducing Bacteria, Lateritic Ore, Goethite, Magnetite, Magnetic Properties
Cite this paper: N. Pradhan , R. R. Nayak , D. K. Mishra , E. Priyadarshini , L. B. Sukla , B. K. Mishra , "Microbial Treatment of Lateritic Ni-ore for Iron Beneficiation and Their Characterization", World Environment, Vol. 2 No. 6, 2012, pp. 110-115. doi: 10.5923/j.env.20120206.01.
Article Outline
1. Introduction
- In lateritic nickel ore, nickel is associated with iron phase where as cobalt is associated with manganese phase[1]. Natural Fe(III) oxides are high in surface area and are reactive[2]. Fe(III) oxides adsorb a wide range of metal cations and anions by complexation to surface hydroxyl groups[3]. Nickel lateritic ore of Sukinda, Orissa, contains 0.8% Ni and 0.049% Co which makes them the only nickel deposit in India. More focus had been given by IMMT Bhubaneswar on this material for extraction of nickel and cobalt[4-7]. However, thermal activation or thermal reduction of ore is required for better recovery of metal values[8]. Heating of ore resulted in conversion of goethite to hematite, which was responsible for releasing nickel from Fe(II) lattice and resulted in better recovery[7]. This type of reduction is not cost effective in terms of energy consumption and may not be employed for large scale operations. Hence, it is desirable to have an alternative ecofriendly and low cost method, which makes the subsequent metal extraction more feasible. Ferric iron oxides are widespread in anoxic aqueous environment and have been reported to act aselectron sink during biodegradation of various natural and xenobioticcompounds[9-11].Microbial Fe (III) reduction results in the generation of several important Fe(II)-containing minerals in sedimentary environments, including magnetite Fe(II)Fe(III)2O4, which is a magnetic mineral. Magnetite formation during dissimilatory Fe(III) reduction is reported for different pure cultures of bacteria[12] as well as for Fe(III)-reducing enrichment consortium[13]. Microbial dissimilatory Fe(III) reduction plays an important role in the geochemical cycling of iron and organic matter in anoxic ecological system[14]. Iron-reducing microbes generally belong to the genera Shewanella, Geobacter, Geovibrio, Desulfobulbus etc [15-17]. If bacterial reduction method is successful, cost intensive method of ore roasting process can be eliminated. Such ‘dissimilatory’ processes have opened up new and fascinating areas of research with potentially exciting practical applications.Applying a biological approach to address this problem, we investigated the use of naturally occurring iron reducing bacteria for the reduction of lateritic nickel ore (iron phase) in a low cost ecofriendly way.
2. Materials and Methods
2.1. Lateritic Nickel Ore
- Lateritic Nickel ore was procured from Sukinda mines of Orissa Mining Corporation (OMC), India. The sample was a low grade lateritic ore which contains about 0.8% Ni, 0.049%Co, 1.92% Cr, 0.32% Mn, and 50.2% Fe. For the experimental purpose the ore samples were heated at 600 ℃ for 5 hours to convert the goethite to hematite which is reported to aid the release of the nickel bound to goethite matrix. Thermal activation of the lateritic nickel ore has significant influence on nickel and cobalt recovery[18]. The thermal activation changes the mineral structure and brings about mineral phase transformation by dehydroxylation of the goethite[19].
2.2. Microorganisms
- Soil sediment from a wetland sites around Bhubaneswar, Orissa, India was used as source of iron reducing bacteria. After collecting, these were immediately transferred to anaerobic vials and taken to the laboratory for subsequent enrichment of Fe(III) reducing bacteria.
2.3. Enrichment and Isolation of Fe(III)-Reducers
- One gram of sediment was transferred to a vial containing 10 ml of anaerobic saline solution. These vials were thoroughly mixed, allowed to settle for 10 minutes and then 5 ml of liquid was used to inoculate 100 ml selective alkaline anaerobic iron reducing medium in volumetric flasks. This medium contains Nickel ore 1.0 g/l; K2HPO4 3.0 g/l; KH2PO4 0.8 g/l; KCl 0.2 g/l; NH4Cl 1.0 g/l; MgCl2 0.2 g/l; CaCL2 0.1 g/l; Yeast extract 0.05 g/l; mixture of vitamins and trace minerals solution 1% (v/v) and 10 mM sodium acetate (C2H3O2Na). pH of the media was adjusted to 7.2 with the help of 1N NaOH. Liquid surface was covered with paraffin oil to make the system anaerobic. The flasks were incubated at 30 oC for several days under dark condition. The enrichment procedure was repeated four times with the transfer of 10 ml of culture as inoculum into the fresh medium. The enrichment cultures after five successive transfers were used as inoculum for lateritic nickel ore reduction experiments.
2.4. Treatment of Lateritic Nickel Ore with IRB Consortium
- Lateritic nickel ore was treated with well enriched Fe(III) reducing bacterial consortium in an anaerobic, alkaline medium at 2% pulp density and kept at 30℃ for 7 days under dark condition. Simultaneously control experiments without any bacterial solution were also run. After treatment, the treated lateritic nickel ore was filtered and air dried. In a similar set of experiments the heated lateritic nickel ore was taken instead of the original ore. The reduction of highly insoluble Fe(III) oxides resulted in dissolution of soluble Fe(II) ions in the growth medium. This soluble ferrous-ion was determined by titration[20].Samples are designated as O1 for lateritic ore and O2 for lateritic nickel ore sample treated with IRB consortium. Similarly R1 for heated lateritic nickel ore sample and R2 for heated lateritic nickel ore sample treated with IRB consortium.
2.5. Analysis
- X-ray diffraction analysis was carried out by an X-ray powder diffractometer (Philips X’pert Pro, Panalytical) using Mo Kα (λ= 0.7107 Å) as X-ray source and a programmable divergence slit. The voltage and current of the x-ray source were 40 kV and 20 mA, respectively. UV-visible absorption spectra of all the samples were taken with a Varian Cary 100 spectrophotometer in the region 200 - 800 nm to determine the mineral species through absorption band gap. Fourier transform infrared spectra were recorded on a FTIR system (spectrum GX model supplied by Perkin Elmer instrument, USA). The spectra were recorded from 400 to 4000 cm-1. The surface morphology was observed in a Field Emission Scanning Electron Microscope (Zeiss Supra 55, observation conditions V—20 kV, I—0.6 nA) after coating the surface with gold to reduce the charging effect. Microscopic image of the IRB consortium was taken in Nikon 80i optical microscope. The room temperature magnetic hysteresis studies of samples were carried out using Vibrating Sample Magnetometer (VSM) (Model: Lakeshore 7410) at an applied magnetic field of 2T.
3. Results and Discussion
- Dissimilatory iron reducing bacterial consortium was enriched in an anaerobic mineral salt medium containing lateritic nickel ore composed of insoluble ferric iron in the form of goethite. The medium contained acetate ions as carbon source (electron donor) and ammonium chloride as nitrogen source for bacterial metabolism. In this system, bacteria oxidizes carbon source and the released electron pass through a series of electron carrier molecules to generate ATP, the biochemical energy needed for bacterial metabolism. The ultimate electron acceptor or electron sink in absence of oxygen is the Fe(III) present in goethite. After accepting the electron, Fe(III) gets reduced to Fe(II) which results in phase change. Due to the phase changes, original light brown colour of the lateritic nickel ore transform to dark brown. No such change was observed in the control experiment.
 | Figure 1. Gram staining of iron reducing bacterial consortia |
 | Figure 2. SEM image showing needle shaped goethite particles (G), bacterial bio-film (BF), Iron reducing bacteria (IRB) and granular magnetite particles (M) in close association with ore particles |
 | Figure 3. Release of Fe (II) ion in medium and change in pH during growth |
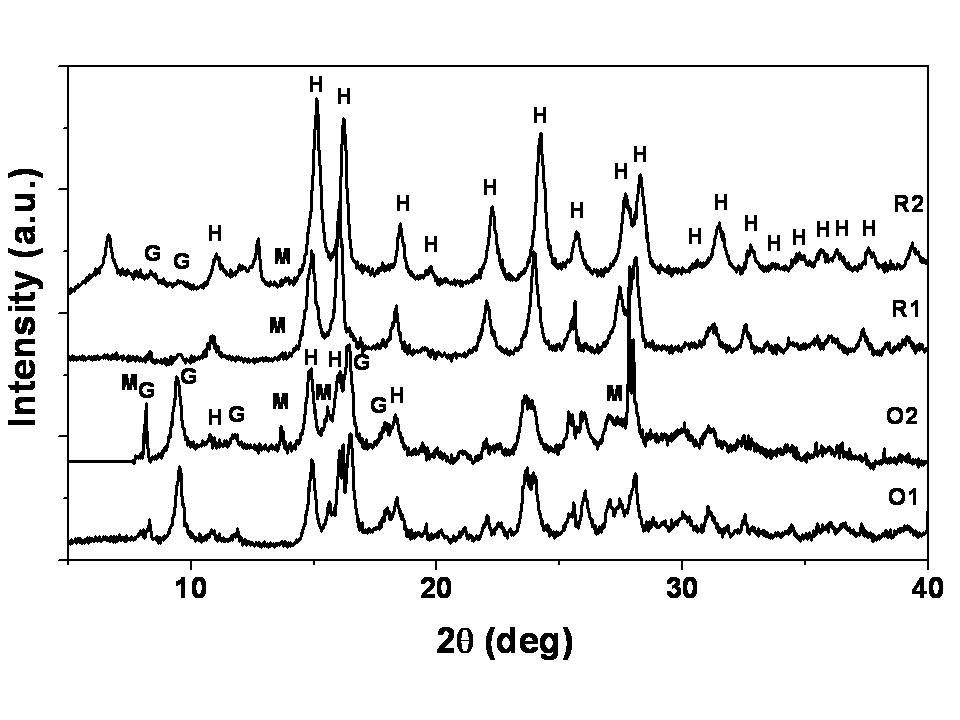 | Figure 4. XRD pattern of original nickel laterite ore and IRB treated samples. H: Hematite (Fe2O3); G: Goethite (FeOOH); M: Magnetite (Fe3O4) |
 | Figure 5. UV-visible spectrum of original nickel laterite ore and IRB treated samples |
|
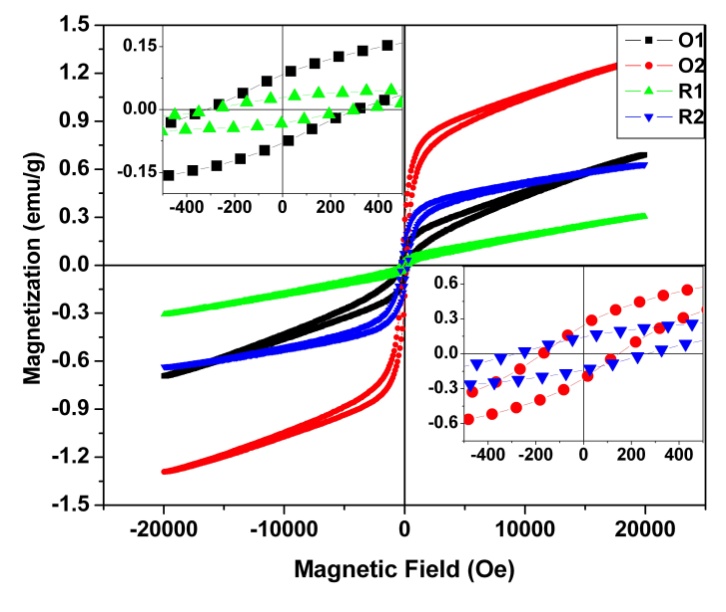 | Figure 6. Hysteresis loop curve for all the samples |
|
4. Conclusions
- Lateritic nickel ore when subjected to treatment with anaerobic dissimilatory iron (III) reducing bacterial consortium show phase changes in iron minerals. Comparison of properties of thermally and microbially reduced lateritic ore vis a vis original ore show that changes in phases brought about by microbial reduction were different than those by thermal reduction. Magnetite formation was more prominent in case of microbial treatment of original ore than roasted ore which is well pictured in the XRD pattern. Roasting leads to the formation of hematite. The carrier mediated exchange interaction between Fe(II) and Fe(III) ions in laterite nickel ore sample treated with IRB consortium is responsible for higher ferromagnetic ordering.IRB consortia treated ore showed higher saturation magnetization value of 1.5 emu/g with remnant magnetization value of 0.328 emu/g and coercivity of 235 Oe. The thermal activation of the same sample shows lowering in ferromagnetic ordering due to the decreasing percentage of Fe(II) and Fe(III) ions. Thermally activated ore was less amenable to further phase changes by microbial treatment. From this study we conclude that naturally occurring iron reducing bacterial consortia could be for better recovery of metal values in a cost effective and eco-friendly way.
ACKNOWLEDGEMENTS
- SEM and XRD measurements were carried out by Mr. Debadutta Sahoo and Mrs. Swagatika Mohanty, to whom our sincere thanks are due. DKM would like to express his sincere thanks to Council of Scientific and Industrial Research (CSIR), India for fellowship under CSIR quick hire scheme. EP would like to express her sincere thanks to Department of Science and Technology, India for ‘Inspire’ fellowship.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML