-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2012; 2(3): 44-50
doi: 10.5923/j.env.20120203.05
Cadmium Uptake, Growth and Phytochelatin Contents of Triticum Aestivum in Response to Various Concentrations of Cadmium
Sonya Hentz , Jacqueline McComb , Gloria Miller , Maria Begonia , Gregorio Begonia
Plant Physiology/Microbiology Laboratory, Department of Biology, P.O. Box 18540, College of Science, Engineering and Technology, Jackson State University, 100 Lynch Street, Jackson, Mississippi 39217, USA
Correspondence to: Gloria Miller , Plant Physiology/Microbiology Laboratory, Department of Biology, P.O. Box 18540, College of Science, Engineering and Technology, Jackson State University, 100 Lynch Street, Jackson, Mississippi 39217, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Cadmium (Cd) contamination of the environment is a major concern because of its impact on human health, food supply chain, and ecosystems. Phytoremediation has emerged as an alternative technology to expensive engineering techniques. The objectives of this research were to evaluate the growth and cadmium uptake of wheat (Triticum aestivum L. cv TAM 109) plants exposed to Cd at different periods; and assess whether phytochelatin (PC) synthesis can explain the wheat’s tolerance mechanism to cadmium. Wheat seeds were grown in sand medium for 30 days (pre-metal treatment). After initial exposure to different Cd concentrations using hydroponic systems, plants were harvested at different day intervals, separated into roots and shoots, dried at 75℃ for 2 days, weighed for dry biomass, and acid-digested for cadmium uptake determinations. Cadmium uptake and PC contents of roots and shoots were quantified using established procedures. Results showed that shoot and root biomass increased with exposure time, and was more pronounced in the shoot than in the roots. Shoot Cd uptake increased with increasing Cd concentrations, except at days 8, 12, and 15 wherein Cd uptake was very minimal especially at 20 µM. Root Cd uptake increased with increasing exposure periods and Cd concentrations. The syntheses of PC in shoots were significantly enhanced only at 20 µM Cd. There were no significant differences in quantity of roots’ PC regardless of Cd treatments.
Keywords: Cadmium, Phytochelatin, Phytoextraction, Triticum aestivum
Article Outline
1. Introduction
- Phytoremediation is the use of living plants for the removal of contaminants from contaminated soil, water, sediments, and air. Soil contamination by heavy metals (HMs) is an important environmental concern because these heavy metals are often persistent in the soil, due to their immobile quality[1]. In minute amounts, many of these heavy metals are essential to sustain life. However, in larger amounts, they become toxic to the environment. The addition of phosphorus fertilizers that often contain a variety of metals especially Cd, is largely responsible for the increasing Cd capacity in agricultural soils[2]. Cadmium in the environment has the capability to bioaccumulate in the body. Cadmium may assemble in biological structures and becomes a substantial health hazard. Prolonged exposure to metals such as cadmium could cause acute and chronic diseases including acute gastrointestinal, respiratory, heart, brain and kidney damage[3]. Phytoremediation is an emerging technology that is correlated indirectly to human health, the food supply chain, and the aquatic and terrestrial ecosystem. The transmission of metals from waste products to the soil and afterwards to plants pose potential danger in which immediate action needs to be taken to eradicate the problem[1]. Generally, heavy metals also impact soil productivity and these contaminated soils are costly to remediate by conventional methods (e.g., removal and burial, or isolation). The major pathway in which heavy metals enter the food supply chain is through plant uptake via the root system. Using plants (phytoremediation) as a clean-up strategy has emerged as a cost-effective and environmentally benign alternative method[4].A major aim of current phytoremediation research is to identify metal-tolerant plants that are able to uptake and translocate heavy metals to their above ground parts (shoots) for easier harvesting[5]. The distribution of heavy metals from the environment to plants for phytoextraction depends on three factors: the total concentration, the bioavailability of elements in the soil solution, and the rate of the element transfer from solid to liquid phases and then to the plant roots[3]. Heavy metal stress can cause direct and indirect consequences to biological processes in plants. The detoxification mechanisms of heavy metals modify catalytic functions of enzymes, and damage cellular membranes[3]. These changes in plants may initiate several preliminary effects, such as inhibition of photosynthesis, mineral nutrient accumulation, root and shoot growth, demineralization, as well as hormonal imbalance and water stress[3]. There is a difference in accumulation of heavy metals depending on their biochemical actions and metal concentrations even for plants of the same species[6]. The success of phytoextraction techniques depends upon appropriate plant species that can accumulate heavy metals and yield large amounts of biomass[5]. Even though total plant biomass is an important factor for phytoextraction, preferential metal accumulation in plant shoots is of utmost importance, therefore the use of hyperaccumulators is significant for phytoextraction of heavy metals in polluted soils.Phytochelatin (PC) synthesis is one of the responses exhibited by plants exposed to HMs, and it is a useful biomarker, reflecting plant’s actual exposure to excess internal HM content[7-9]. A key question however, is whether there has to be a critical minimum limit of heavy metal in plants, before PCs start to form. Also, the use of chelating agents has been proposed to achieve higher removal rates of metals. The use of these synthetic chelators such as Ethylenediamintetraacetic acid (EDTA), can pose environmental concerns because of their high solubility and persistence in the soil. However, Ethylenediamine- N,N'-disuccinic acid (EDDS), an EDTA isomer is easily biodegrable and has less deleterious effect on the environment[10].Wheat (Triticum aestivum) is the plant of primary importance chosen for this research. It is a monocot plant whose stems contain various cells (parenchyma, and sclerenchyma) that aid in the capacity of the plant to withstand stress factors[11]. These types of plants also exhibit an intercalary meristem that contributes to the stem strength[11]. Wheat plants may also be grown year round and during the colder seasons which suggest that these plants can withstand harsh environmental conditions. Wheat has a fibrous root system that helps hold soil particles together and aid in the accumulation of heavy metals. While most metals have toxic effects and act as persistent pollutants in the environment, cadmium and lead are metals of primary importance[12] and are ranked second and seventh, respectively, on the Comprehensive Environmental Response Compensation and Liability Act (CERCLA) priority list of hazardous substances due to their wide spread distribution, availability, and toxicity[13]. This study was conducted to: 1) evaluate the growth and Cd accumulation by wheat (Triticum aestivum L. cv TAM 109) plants exposed to various levels of Cd (supplied as cadmium nitrate) at different growth periods, 2) determine whether wheat (Triticum aestivum L.) plants can tolerate and translocate high concentrations of cadmium, and 3) assess phytochelatin (PC) synthesis in Triticum aestivum L. exposed to Cd.
2. Materials and Methods
2.1. Growth Conditions
- Wheat (Triticum aestivum L. cv. TAM 109) seeds (72 seeds/pan) were sown in 42.2 cm x 30.2 cm x 6.7 cm Jiffy- Foil pans containing sand (Showscape Play Sand). Germinated seedlings were maintained for 30 days at the Jackson State University greenhouse. During this 30-day pre- treatment period, the plants were watered with tap-water twice a day (once in the morning and again in the afternoon). After the 30-day growth period, the seedlings were acclimated by irrigating them with 40 mL of modified Hoagland’s nutrient solution, which contained the following nutrients in mM: calcium chloride dihydrate, 2.0: potassium orthophosphate mono-H, 0.125: magnesium sulfate heptahydrate and potassium sulfate, 0.50: Fe sequestrene, 10.0: ammonium nitrate, 2.50; the following are in µM: boric acid, 2.30: manganous sulfate monohydrate, 0.9: zinc sulfate heptahydrate, 0.60: cupric (copper II) sulfate pentahydrate, 0.15: sodium molybdate dihydrate, 0.10: cobalt (II) chloride hexahydrate, 1.0: nickel chloride hexahydrate, 0.10.During the 30-day growth period, each pan was rotated counter clock wise to ensure that all plants received adequate amounts of sunlight (i.e. no positional effects). After the plants were allowed to go through their vegetative stage, plants were selected for uniformity. Sand particles adhering to the roots of each plant were carefully washed with tap water. Clean plants (4 plants/600 mL styrofoam cup) were placed in designated dilute solution of cadmium nitrate (0, 0.1, 1.0, 5.0, 10.0, 15.0, 20.0 µM Cd). Each plant was secured by wrapping its stem base with cotton to ensure that the plants remained upright with the roots fully immersed in the solution. Plants were irrigated with modified Hoagland’s solution as needed to replenish the volume lost through evapotranspiration. Cadmium mixtures were agitated daily to make sure the metal remained suspended in the solution.The plants were harvested at designated intervals (e.g., 0, 2, 5, 8, 12, 15 days) after treatment initiation. Plants for time 0 were allowed to remain in the metal mixture for 6 hours and then removed. The remaining times where harvested according to their respective days. The harvested plants were rinsed with deionized water to remove any metal that may have been adhering to the root epidermis. They were then towel blotted and placed in a specimen cup. Two plants were harvested for phytochelatin analysis. The roots and shoots where separated and segregated parts (roots and shoots) of each plant were placed in a 15 mL BD Falcon tubes and placed in a Thermo Electron Corporation Forma -80℃ ULT Freezer. The remaining two plants were separated into roots and shoots and analyzed for morphological characteristics (biomass and length, length data not shown).
2.2. Acid Digestion
- The segregated plant parts were placed in corresponding labeled brown paper bags and dried in a Precision Mechanical Convection Oven at 70℃ for at least 48 hours. Dried samples were weighed in a Mettler AE 260 Delta Range to obtain the biomass. Cadmium contents from plant tissues were extracted using a modified nitric acid-hydrogen peroxide procedure according to U.S. Environmental Protection Agency[14] EPA SW-846 Method 3040B. Approximately 100-200 mg of dried tissue plant samples were placed in a 250 mL Erlenmeyer flask and 40 mL of 50% nitric acid were added and allowed to set overnight. Samples were placed on a hot plate (high setting) and refluxed for 15 minutes until a yellow liquid appeared. The solutions were allowed to cool for 10 minutes and 10 mL of 50% nitric acid (HNO3) were pipetted into each sample. The samples were refluxed for 30 minutes and then allowed to cool for 10 minutes. The samples were completely dissolved in 5 mL of concentrated nitric acid. The oxidized solution was allowed to cool and 2 mL of deionized water and 3 mL of 30% hydrogen peroxide were added. The digested solution was heated until the effervescence diminished. Another 7 mL of 30% hydrogen peroxide were added in 1 mL intervals and the digestate was heated again and reduced to approximately 5 mL. After heating, the solution was diluted to 20 mL with deionized distilled water (ddw) and the digestate was filtered through filter paper (Whatman No. 1) and the final volume was adjusted to 25 mL with ddw. Cadmium contents of each sample were quantified using Inductively Coupled Plasma-Optical Emission Spectrometry (Perkin Elmer Optima 3300 DV).
2.3. Phytochelatin Synthesis Analysis
- According to Keltjens and Van Beusichem[15] plant parts (roots or shoots) were homogenized separately for 5 minutes in 10 mL of extraction mixture with a mortar and pestle on ice under nitrogen gas to prevent oxidation. The homogenates were placed in centrifuge tubes and cooled on ice. When twelve tubes filled with wheat homogenate were collected, they were centrifuged in a Beckman Optima XL 100K Ultra Centrifuge for 15 minutes at 10,000 revolutions per minute (rpm) at 4℃. The supernatant was then decanted into a 15 mL BD Falcon tube. Phytochelatin concentrations were determined indirectly by assessing the absorption of total acid-soluble thiols, total glutathione, and oxidized (GSSG) glutathione. The total acid-soluble thiols were determined using Ellman’s reagent[16]. Total glutathione and oxidized glutathione were established using GSSG recycling method[17].
2.4. Statistical Analysis
- The data were analyzed using Statistical Analysis System (SAS V9). Treatment comparisons were done using Fisher’s Least Significant Difference (LSD) test (p < 0.05).
3. Results and Discussion
- Shoot biomass analysis revealed that there was a decrease in biomass as the concentration of cadmium administered was amplified as shown in Figure 1. With regard to days after initial exposure to the metal, the shoot biomass increased as exposure time increased. Plants exposed to 20 µM Cd showed the greatest inhibition of shoot biomass. Plants exposed to Cd for 5, 8, 12, and 15 days were significantly affected as compared to the control (p ≤ 0.016). Shoot biomass followed a dose response trend with day 15 showing the greatest level of toxicity at 20µM. Days 5 and 8, 0.0468 ± 0.0095 and 0.0616 ± 0.0126; days 2 and 5, 0.0373 ± 0.0116 and 0.0468 ± 0.0095; days 0 and 2, 0.0303 ± .0091 and 0.0373 ± 0.0116 g/plant respectively were not significantly affected as compared to each other. Day 15 demonstrated the greatest shoot biomass increase (0.1130 ± 0.0310 g/plant). There were similar trends in shoot biomass for increase in treatments and growth periods. Plant shoots exposed to cadmium nitrate at 2 and 5 days after initial exposure remained within the same biomass range, 0.0373 ±0.0067 and 0.0468 ± 0.0055 g/plant, respectively. Day 8 showed a fluctuation in shoot biomass with an increase for 0.1 µM (0.0794 ± 0.0050 g/plant) and then a slight decrease at 1 and 5 µM (0.0635 ± 0.0044 and 0.0613 ± 0.0040 g/plant), respectively, followed by an increase at 10 µM (0.0716 ± 0.0041g/plant) and lastly a decrease for 15 and 20 µM (0.0404 ± 0.0081 and 0.0462 ± 0.0102 g/plant), respectively. Days 12 and 15 showed a decrease in shoot biomass as the concentration increased and also as exposure time increased.
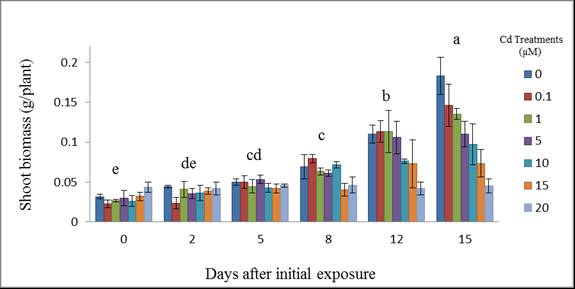 | Figure 1. Wheat shoot biomass (g/plant) at 15 days after initial exposure to Cd. Treatment means with common letters do not differ significantly from other days (p ≤ 0.05) |
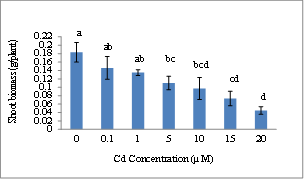 | Figure 2. Wheat shoot biomass (g/plant) at 15 days after initial exposure to Cd. Treatment means with common letters do not differ significantly from each other (p ≤ 0.05) |
 | Figure 3. The morphology of Triticum aestivum L. plants at 15 days after initial exposure to different Cd (µM) treatments |
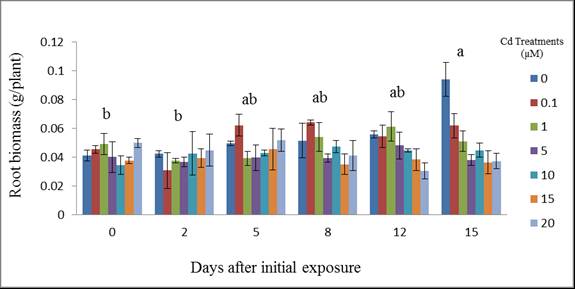 | Figure 4. Wheat root biomass (g/plant) at different days after initial exposure to Cd. Day means with common letters do not differ significantly from other days (p ≤ 0.05) |
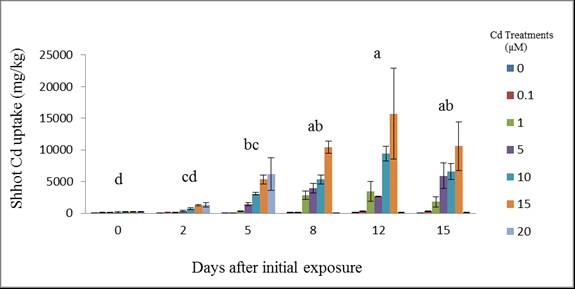 | Figure 5. Cadmium uptake by wheat shoots at different days after initial exposure to Cd. Day means with common letters do not differ significantly from other days (p ≤ 0.05) |
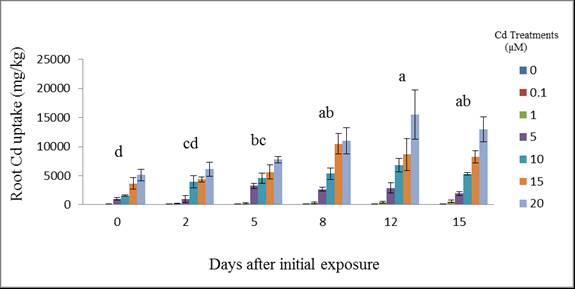 | Figure 6. Cadmium uptake by wheat roots at different days after initial exposure to Cd. Day means with common letters do not differ significantly from other days (p ≤ 0.05) |
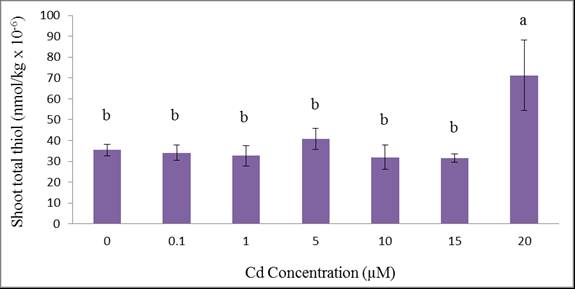 | Figure 7. Total acid-soluble thiols of wheat shoots grown for 12 days at different Cd concentations. Treatment means with common letters do not differ significantly from each other (p ≤ 0.05) |
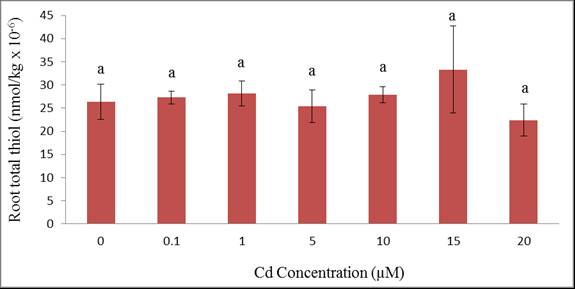 | Figure 8 Total acid-soluble thiols of wheat roots grown for 12 days at different Cd concentrations. Treatment means with common letters do not differ significantly from each other (p ≤ 0.05) |
4. Conclusions
- The experiment suggests that wheat is an efficient Cd accumulating plant. All plants survived and were tolerant to cadmium except those exposed to 20 µM Cd as exhibited by their phytotoxic symptoms. Metal accumulation were not only sequestered in the roots but also translocated to the shoots suggesting that wheat plants are good accumulators of cadmium. We can conclude that the translocation of Cd from root to shoot is through the xylem which is the main process in shoot accumulation. Although wheat plant growth and uptake were affected, the plants demonstrated tolerance to cadmium contamination which may aid in their colonization in cadmium-polluted environments. It appeared that total thiols which represented phytochelatin synthesis can partially explain the tolerance of wheat to Cd. Such phytochelatin synthesis was triggered at a relatively high Cd treatment (20 µM) which was more evident in shoot rather than in the root.
ACKNOWLEDGEMENTS
- This publication was made possible by support provided by the U.S. National Aeronautics and Space Administration (NASA) through The University of Mississippi (UM Subcontract No. 11-08-010 to Jackson State University; M.Begonia. P. I.) under the terms of Agreement No. NNG05GJ72H. The opinions expressed herein are those of the authors and do not necessarily reflect the views of NASA or The University of Mississippi. We acknowledge the partial financial support of the Department of Biology, CSET, JSU.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML