-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2012; 2(2): 11-15
doi: 10.5923/j.env.20120202.03
Prevalence of Legionella Pneumophila in Production Networks and Distribution of Domestic Hot Water in Morocco
Mariam Mekkour 1, 2, El Khalil Ben Driss 2, Nozha Cohen 1
1Division de Microbiologie et d’Hygiène des Produits et de l’Environnement, Institut Pasteur du Maroc, Casablanca, 20360, Maroc
2Département de Biologie, Faculté des Sciences-Université Abdelmalek Essaadi, Tétouan, 93000, Maroc
Correspondence to: Nozha Cohen , Division de Microbiologie et d’Hygiène des Produits et de l’Environnement, Institut Pasteur du Maroc, Casablanca, 20360, Maroc.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
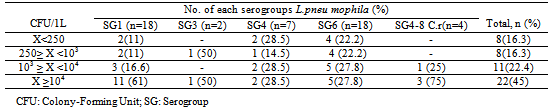
The goal of this study was to evaluate the prevalence of Legionella pneumophila specie in hot water samples from tanks and ecological networks of production and distribution of domestic hot water in Morocco. To accomplish this study, 156 samples of hot water, were collected from thirty four structures’ from January 2009 to December 2010, were analyzed to search and enumeration of Legionella pneumophila by culturing onto a selective medium. The results of all samples showed that Legionella pneumophila was detected in 31.5% of total samples analyzed 67.4% with a load above the limit tolerated by the regulations. The serological survey showed that 37% of isolates are Legionella pneumophila serogroup 1 while 63% correspond to serogroup 2-15 whose serogroup 6 was the most frequently isolated (37%). The results of this study show that Morocco has a high frequency in Legionella pneumophila serogroup 1, which is primarily responsible for the majority of Legionnaires’ disease, which will encourage the authorities to take necessary measures for the surveillance and preventive actions for monitoring of the disease legionellosis in Morocco.
Keywords: Aerosol, Biofilms, Hot Water, Legionnaires’ Disease, Legionella Pneumophila
Article Outline
1. Introduction
- Legionella pneumophila, the agent responsible for Legionnaires’ disease and Pontiac fever[1], was first discovered after a large outbreak in an American hotel in 1976. This bacterium has been found in various aquatic environments, e.g., ponds, rivers, lakes[2] as well as in soil and his survival depend upon several factors, such as the presence of protozoa[3], heterotrophic bacteria, pH, temperature, oxygen level, and plumbing fixture materials[4]. L. pneumophila, for which 14 serogroups have been identified, is the species most commonly associated with disease outbreaks[5]. L. pneumophila does not pose a serious health risk due to its low amounts in natural habitats. However, man-made systems serve as amplifiers for L. pneumophila by providing suitable conditions for growth and multiplication[6]. Numerous epidemic investigations have convincingly demonstrated that domestic hot water systems have served as sources of legionellae infection[7]. Frequently, legionellae has been isolated in high numbers from water, sediments[8] and slimes in cooling water systems[6]. Contaminated aerosols, acquire the infection and inhalation of these aero- sols presumably produces Legionnaires’ diseas[9], but Nointerhuman transmission has yet been found[10]. In this study, the existence and distribution of L. pneumophila were investigated to find the sources of previously reported sporadic cases of Legionnaires’ disease in Morocco. The goal of this study was to evaluate the frequency of colonization by Legionella pneumophila in hot water systems of the different structures’ in Morocco.
2. Materials and Methods
2.1. Sample Collection
- From January 2009 through December 2010, a total of 156 water samples were collected from 26 hotels, 6 factories and 2 gyms of nine towns representative of different Moroccan regions. The first jet of hot water was drawn from the bathroom outlets (showers or taps) and placed in sterile bottles. In order to neutralize the residual free chlorine, 10% sodium thiosulphate was added in sterile bottles for bacteriological analysis (1 ml /L). Only sterile bottles were employed throughout the study. Water samples were taken to the laboratory and immediately processed.
2.2. Microbiologic Analysis
- To detect Legionella pneumophila, 1-L water samples were concentrated by sterile cellulose nitrate membrane filters whose a pore size is 45µm (Sartorius AC, Goettingen, Germany). The intact membranes were aseptically removed, placed into sterile 5 mL screw-capped containers. Each concentrated water sample was sonicated for tow min to dislodge bacterial cells from the membranes using a model B2 210-DTH ultrasonic cleaner (Danbury, USA). 2 ml of the suspension was pre-treated with heat (50ºC water bath during 30 min) and the other 2 ml was pre-treated with acid (2ml of HCl-KCl solution, pH 2.0). The heat and acid pre-treatment of concentrated water samples were used as a selective method to reduce the numbers of non-Legionella bacteria as previously described[11,12]. Plates containing selective medium for growth and isolation of Legionella-CYE- agar (base) supplemented with BCYE α growth-supplement and GVPC selective supplement (Legionella-Combi-Pack, Merck E, Darmstadt, Germany) were inoculated with a 0.1 ml of hot pre-treatment sample and with a 0.2 ml of acid pre-treatment sample. All the samples were incubated at 35°C ± 2°Cwith 2.5% CO2 in a humid atmosphere for 10 days and examined at 3, 5, and 10 days. Colonies suspected of being of Legionella were subcultured on BCYE and on blood agar plates; those that failed to grow on the blood agar plates but grew on BCYE with cysteine were identified by the latex agglutination method, which also allowed us to distinguish serogroup 1 from serogroups 2–14 (Slidex Legionella- kit, BioMérieux, Lyon, France). Our positive control was an antigen suspension of L. pneumophila 1 (ATCC 33152) obtained from a separate culture. For L.pneumophila serogroup subclassifications 2, 3, 4, 5, 6,.., 14 were identified by the agglutination technique of latex particles sensitized with monoclonal antibodies (reagent supplied by BioMérieux).
3. Results
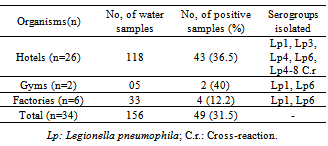
- Of the 156 samples analyzed above, 118 came from hotels, 33 from Factories and 5 from gyms of different areas in Morocco; the results of all samples were negative for Legionella only in 13 (38.2%) structures’. Legionella pneumophila were detected in 49 samples (31.5%), 43 (36.5%) of which were from hotels, 2(40%) of which were from gyms and 4 (12.2%) of which were from Factories (Table 1).
|
|
4. Discussion
- Legionella are readily found in natural aquatic bodies with some species been recovered from soil[13,14]. Studies have shown that Legionella species are present in all segments of community water supplies, water treatment structures’[15]. The aquatic environment of this bacteria also includes man made habitats such as cooling towers, evaporative condensers, whirlpool spas, decorative fountains, air conditioner water systems, and potable-water distribution systems, so the colonization of this artificial habitats by L. pneumophila depends on a combination of several factors, including water temperature, sediments accumulation, and commensal microflora[16,17]. Bacterial transmission to humans is most commonly accepted to be via droplets generated from these environmental sources. The epidemiological evidence suggests that the aerosol travels and remains infective from several hundreds meters to a few kilometers[18]. Our study showed that Morocco is not exempted from the list of countries where Legionella pneumophila is found (the most virulent species of Legionella), this bacteria has been isolated from a wide variety of water types, such as potable water of hotels, factories and gyms. Moreover, several authors have described the isolation of this bacteria from showers, cooling towers and boilers[11,19], which is in agreement with the findings of the present study.The use of selective media like BCYE agar medium has proven to be very important in the isolation of Legionella and L. pneumophila in this study as demonstrated by other researchers in this field[20,21]. Our study also showed that the medium alone was not sufficient for the selective isolation of L. pneumophila because environmental organisms seemed to come through despite the incorporation of antibiotics that selectively inhibit the growth of competing microflora of the environments including fungi[13]. This study is in line with other studies where the BCYE with antibiotics was used in the detection and isolation of Legionella from the environments[13,22].
|
5. Conclusions
- we suggest that clinicians should apply the whole spectrum of laboratory methods for the diagnosis of legionnaires ’ disease and take samples for culture wherever possible, and we recommend that targeted behavioral control messages be directed at the sectors of population in order to warn them against the possible risks of L. pneumophila infection from environmental sources. Routine monitoring of environmental water for L. pneumophila is expected to prove helpful in efforts to reduce the bacterial contamination of water systems and is also expected to facilitate the development of a more active prevention strategy for Legionnaires’ disease. Additionally, further study will require that the focus be kept on correlation analysis by clustering between environmental and clinical isolates of Legionella species. Thus, the findings of this study highlight the importance of understanding the epidemiology and ecology of L. pneumophila from public facilities in terms of public health; in this regard, our findings corroborate and reinforce the recommendations made in several previous studies[33,34].
ACKNOWLEDGMENTS
- We express our gratitude to our coworkers who provided assistance. We also thank Dr. Fabien SQUINAZI (Laboratoire d’Hygiène de la Ville de Paris) and Dr. Sophie JARRAUD (Centre National de Référence de Legionelles, Lyon) for their help to make serotyping of Legionella pneumophila strains.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML