-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2012; 2(1): 1-5
doi: 10.5923/j.env.20120201.01
Mobilization of Metals from Indian Coal Fly Ash under Dynamic Conditions
Deepak Kulkarni 1, Lokeshappa B 1, Kandarp Shivpuri 1, Anil Kumar Dikshit 1, 2, 3
1Centre for Environmental Science and Engineering, IIT Bombay, Mumbai, 400076, India
2School of Civil Engineering, Survey and Construction, University of KwaZulu-Natal, Durban, 4041, South Africa
3School of Civil and Environmental Engineering, Nanyang Technological University, 639798, Singapore
Correspondence to: Anil Kumar Dikshit , Centre for Environmental Science and Engineering, IIT Bombay, Mumbai, 400076, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The toxic metals leaching through coal fly ash has environmental concern. The dynamic ageing study was carried out to understand the behavior of the elements during leaching from coal fly ash. The 48 hours experiments, simulating fly ash under the rain water condition, for determining the metal leaching were performed. The leachates were analyzed through the inductively coupled plasma atomic emission spectroscopy (ICP-AES) for elements Arsenic, Chromium, Copper,Selenium, Nickel, Barium, Vanadium and Lithium. It was found that although variable but significant amount of As, Cr, Se,Ni, Ba, V, and Li leached from nine fly ash samples studied. The amount of as leached ranged from 450-680 ppb. Similarly for Cr, Se, Ni, Ba, V, and Li the amount of metal leached ranged from 10-220 ppb, 2-24 ppb, 10-240 ppb, 20-120 ppb, 10-1100 ppb and 20-750 ppb respectively. The concentration of all the metals leached with copper being an exception, were found to be higher than WHO recommended values for drinking water.
Keywords: Ash Pond, Coal Fly Ash, Leachate, Metals
Cite this paper: Deepak Kulkarni , Lokeshappa B , Kandarp Shivpuri , Anil Kumar Dikshit , "Mobilization of Metals from Indian Coal Fly Ash under Dynamic Conditions", World Environment, Vol. 2 No. 1, 2012, pp. 1-5. doi: 10.5923/j.env.20120201.01.
Article Outline
1. Introduction
- Urbanization and industrialization has enhanced the power consumption, which has ultimately increased the coal fly ash generation. Annually about 700 million tons of coal fly ash is generated all over the world. In India, about 112 million tons of coal fly ash is generated which has occupied 65000 acres of land for ash ponds[1]. Coal used for the Indian thermal power plans has ash content between 25 and 45%[2]. The fly ash utilization rate in India is about 25- 38%[3]. The coal fly ash is being utilized for different purposes. If the difference between generation and utilization rate is marginally increased, then it leads to more land requirement for its disposal.Coal fly ash is generated from thermal power plants as a result of combustion process and it contains numerous toxic substances that cause air, water and soil pollution[4]; disrupt ecological cycles and put forth environmental hazards[5]. The high temperature of burning of coal turns the clay minerals present in the coal powder into fused fine particles mainly comprising of aluminium silicates. The vaporised elements in the boiler furnace get condensed and enriched on the surface of finer ash particles because of volatilisation and condensation mechanism[6,7]. The fly ash carried by flue gas is collected by electrostatic precipitator[8]. The fly ashes contains toxic metals such as As, Ba, Hg, Cr, Ni, V, Pb,Zn, and Se and are characteristically enriched in fly ash particles. The mobility of potential pollutants due to ash leaching has been subject to extensive research[9].In fly ash trace, metals are present in relatively small quantity but they can cause long term effects on the man, plants and animals through air, water and soil as it has cumulative build up, long life and toxicity. Many trace element present in fly ash can leach out and contaminate the soil and water body and groundwater, therefore study in their leaching characteristics and potential has been regarded important for the protection of environment[10].The surface of a fly ash particle is only microns in thickness and can contain leachable heavy metals(i.e. trace elements) which have condensed on to the surface. Inorganic salts make the particle surface more reactive than the glassy matrix. Elements which are leachable from fly ash such as As, B, Ca, Cr, Mg and Sr, are likely to be dominantly partitioned into the exterior glass surface following condensation reactions during combustion; while elements such as Al, Si, K, Pb and many of the other trace elements are distributed throughout the particle and are not preferentially concentrated. When fly ash comes into contact with an aqueous environment some constituents will dissolve to a greater or lesser extent and become mobile. The product of this contact is known as the leachate. The degree of mobilisation and dissolution of the constituents in the leachate is of interest.Leachability study is carried out to determine the behaviour of dumped coal fly ash when exposed to external influences and the way it affects the nearby environment. Leachability is highly variable and depends on the type of coal and combustion conditions. The acidic fly ash produces a higher concentration of metals in the leachate than the alkaline fly ash.The rate of leaching is affected by the fly ash particle size, agitation of the mixture, pH and temperature of the water[11]. pH is the influential parameter for leaching of the metals for example As is in dissolved phase when pH is greater than 7 and comparatively high amount of As leached or desorbed from fly ash[12]. In the present research work, leaching experiments were carried over 48 hours, simulating fly ash under the rain water environment, for determining the metal leaching.
2. Materials and Methods
2.1. Sample Collection
- Fly ash samples were collected from directly from the electrostatic precipitators of the nine thermal power plants situated all over the State of Maharashtra, India These ashes have been labeled as Ash-A to Ash-I. The ashes were sieved finer than 250 µm for further experiments. Fly ashes were characterized in terms of particles size by Beckman Coulter Laser Diffraction Particle Analyzer (LS 13320, Japan)., surface area by BET surface area analyser (Smart Instruments, India), mineralogy by X-Ray Diffraction(XRD) using Phillips Defractometer (PW 1840, Netherland), and metal oxides content by X-Ray Fluorescence(XRF) using Philips X-Ray Fluorescence Spectrometer (2404, Netherlands).
2.2. Sample Preparation
- Ash slurries were prepared by mixing 5 g of each ash and 96.5 mL of rain water with solid to liquid ratio of 1:20 according to TCLP procedure (TCLP-1311, 7.1.4). The rain water having pH of 5.9 was collected as a leachant media during the rains in the month July 2011.
2.3. Experimental Setup
- The ash slurry (5 g of ash and 96.5 mL of rain water) was taken each of a number of 100 mL conical flasks, which were agitated in an orbital shaker (Trishul Equipment, India) at 160-180 rpm. Initial pH of zero hour slurry was also measured, which was not kept in orbital shaker. The pH readings of the supernatant were measured at every 2 hrs interval for first 24 hrs and then at 30, 36 and 48th hrs. The 10 mL supernatant were drawn for each flask, filtered through 0.22µ PTF filter and preserved till ICP-AES analysis by acidifying by 2% nitric acid(by mass). For each sample, pH was measured using Thermo Scientific pH Meter (Orion 3Star, Singapore) and metals leached were quantified using ICP-AES.
2.4. Metal Analysis
- The sample was diluted with 2% nitric acid to a dilution factor of 1:2 prior to ICP-AES analysis.
3. Results and Discussions
- The results of coal fly ash characterization, pH and metals leached from coal fly ash samples under dynamic condition are discussed in following sections.
3.1. Characterization of Coal Fly Ash
3.1.1. Mineralogical Composition
- The XRD results of raw coal fly ashes are shown in the Figure 1. The mineralogical composition shows that Mullite and Quartz are present in all fly ash samples except Ash-B, which is calcium rich fly ash, in which the dissolution of Mullite is observed as shown in Table 1.
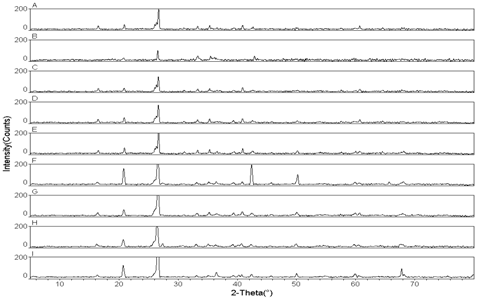 | Figure 1. Pattern of XRD for ash samples Ash-A to Ash-I |
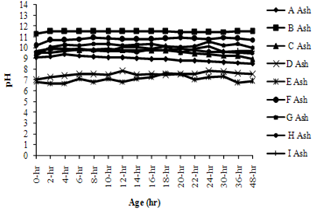 | Figure 2. Variation of pH of coal fly ashes with age |
3.1.2. Particle Size and Surface Area
- Particle size analyses were based on laser diffraction, particle passing through a lesser beam scattered light at an angle that was directly related to their size. As particle size decreased, the observed scattering angle increasedlogarithmically. The nine fly ashes from thermal power plans were also analyzed for surface area. The particle size ranged from 2.14 µm to 207 µm, whereas surface area ranged from 0.65 m2/g to 1.2 m2/g which is shown in Table 1.
3.2. Variation of pH
- The variation of pH with the time for all nine coal fly ashes is shown in the Figure 2. Initially, pH value increases above the 9 in case of Ash-A, B, C, F, G, H and I whereas pH values for Ash-D and Ash-E were found to be in the range of 7. The sudden rise in pH was due the dissolution of CaO in the case of calcium rich ash (Ash-B) whereas for the remaining ashes, the dissolution of silica and aluminous oxides caused sudden rise in pH.
3.3. Metals Mobilized from Fly Ashes
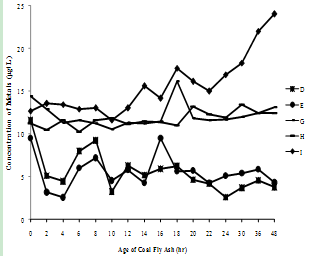
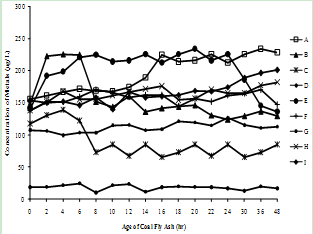
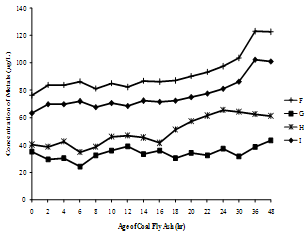
- The supernatants of all nine fly ash samples were analyzed for the variation of metal mobilized with time varying from 0 to 48 hr. The concentrations of various metals mobilized from various ashes are shown in Figure 3(a) to (h).
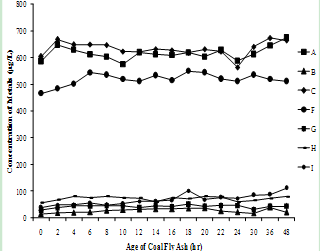 | Figure 3(a). Variation of Arsenic concentration with time |
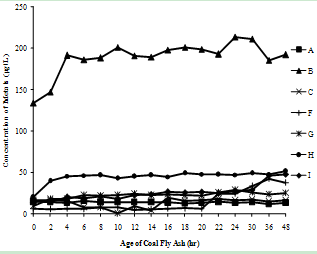 | Figure 3(b). Variation of Chromium concentration with time |
 | Figure 3(c). Variation of Copper concentration with time |
 | Figure 3(d). Variation of Selenium concentration with time |
 | Figure 3(e). Variation of Nickel concentration with time |
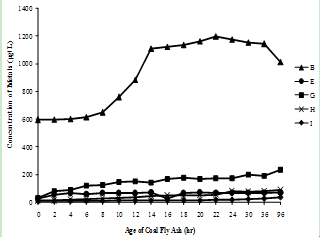 | Figure 3(f). Variation of Barium concentration with time |
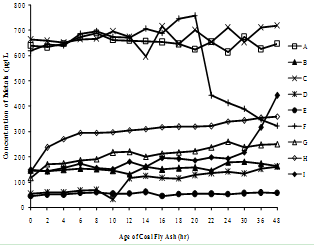 | Figure 3(g). Variation of Vanadium concentration with time |
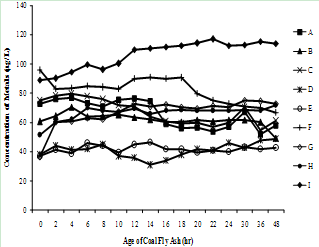 | Figure 3(h). Variation of Lithium concentration with time |
5. Conclusions
- This paper examines the metal mobilization under dynamic condition for nine coal fly ash samples obtained from Indian thermal power plants. Most of the elements such as As, Cr, Se, Ni and V showed concentration in the leachate in 48 hr time above threshold limits whereas copper leached in the minimum concentration. The overall pH of the all the ashes was found to be more than 7 except Ash-E. Based on the mineralogical composition, all the ashes were classified as class F ash except ash-B which was class C ash. Based on the study, following conclusions can be drawn:● pH of all the samples varied from 7.0 to 11.0 i.e mostly alkaline.● Overall, the concentration of metals leached were above the WHO prescribed drinking water standards.● Among the major elements examined vanadium was leached in larger amount.In absence of proper control measures, these leached metals may contaminate the receiving water body including groundwater.
ACKNOWLEDGEMENTS
- The authors are grateful to the Centre for Environmental Science and Engineering for providing ICP-AES facilities for this project; Department of Earth Science for providing XRD and Sophisticated Analytical Instrument Facility(SAIF) for providing XRF all at IIT Bombay, India. The authors are thankful to management and staff of all the nine Thermal Power Plants in Maharashtra, India for providing the fly ash samples used in this research.
References
| [1] | Dhadse, S., Kumari, P., and Bhagia, L. J., 2008, Fly ash characterization, utilization and government initiatives in India - A riview., Journal of Scientific and Industrial Research, 67, 11-18 |
| [2] | Bhattacharjee, U., and Kandpal, T.C., 2002, Potential of fly ash utilisation in India., Energy, 24, 151-166 |
| [3] | P. Asokan, “Application of some inorganic residues in management of hazardous jarosite waste,” Ph. D thesis, Indian Institute of Technology Bombay, India, 2007 |
| [4] | Baba, A., Gurdal, G., Senqunalp, F., and Ozay, O., 2008, Effect of leachant temperature and pH on leachability of metals from fly ash. A case study: can thermal power plant, province of Canakkale, Turkey., Environmental Monitoring Assessment, 139(3), 287-298 |
| [5] | Meij, R., 1994, Trace element behaviour in coal fired power plants., Fuel Processing Technology, 39, 199-217 |
| [6] | Kumari, V., 2009, Physicochemical properties of fly ash from thermal power station and effects on vegitation., Journal of Resources, Conservation and Recycling, 3(2), 102-105 |
| [7] | Roy, A., Eaton, H.C., Cartledge, F.K., and Tittlebaum M.E., 1991, Solidification and stabilization of heavy metal sldge by portland cement/fly ash binding mixture., Hazardous Waste and Hazardous Material, 8(1), 33-41 |
| [8] | Ghuman, G.S., Menon, M.P., Chandra, K., and James, J., 1994, Uptake of multielements by corn from fly-compost amended soil., Water, Air and Soil Pollution, 72, 285-295 |
| [9] | Mattigod, S.V., Rai, D., Eary, L. E., and Ainsworth, 1990, Geochemical factors controlling the mobilization of inorganic constituents from fossil fuel combustion residues: I. review of major element., Journal Environmental Quality, 19, 188-201 |
| [10] | Fytianos, K., and Tsanikidi, 1998, Leachability of heavy metals in Greek fly ash from coal combustion., Environmental International, 24(4), 477-486 |
| [11] | Pandey, V.C., Singh, J.S., Singh, R.P., Singh, N., and Yunus, M., 2011, Arsenic hazards in coal fly ash and its fate in Indian scenario., Resource, Conservation and Recycling, 55(9-10), 819-835 |
| [12] | Praharaj, T., Powell, M.A., Hart, B.R., and Tripathy, S., 2002, Leachability of elements from sub bituminous coal fly ash from India., Environmental International, 27(8), 609-615 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML