-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2011; 1(1): 6-13
doi: 10.5923/j.env.20110101.02
Pollution in Water of Kasardi River Flowing along Taloja Industrial Area of Mumbai, India
Ram S. Lokhande 1, Pravin U. Singare 2, Deepali S. Pimple 3
1Department of Chemistry, University of Mumbai, Santacruz, Vidyanagari, Mumbai 400098, India
2Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai 400058, India
3Department of Chemistry, R.J. College, Ghatkopar, Mumbai 400086, India
Correspondence to: Pravin U. Singare , Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai 400058, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The area of study selected in the present investigation was Kasardi River which receives heavy discharge of waste effluent from the nearby Taloja industrial belt which is one of the fastest developing industrial belt of Mumbai. The study was performed to investigate the concentration of toxic heavy metals like chromium (Cr), cadmium (Cd), nickel (Ni), zinc (Zn), copper (Cu), lead (Pb) and iron (Fe) in river. It was observed that concentration of most of these heavy metals were much higher than the maximum permissible limits. These heavy metals have created threat to the aquatic life and through biomagnifications may enter the food chain thereby affecting the human beings as well. The research work was extended further to study the physico-chemical properties like temperature, pH, solid content, chloride, oil / grease content, BOD and COD values of the river water. The authors point out that as India moves towards stricter regulation of industrial effluents to control water pollution, there is a need to implement common objectives, compatible policies and programmes for improvement in the industrial waste water treatment methods.
Keywords: Heavy Metals, River Water Pollution, Industrial Effluent, Physico-Chemical Properties, Kasardi River, Taloja Industrial Area, Mumbai; India
Cite this paper: Ram S. Lokhande , Pravin U. Singare , Deepali S. Pimple , "Pollution in Water of Kasardi River Flowing along Taloja Industrial Area of Mumbai, India", World Environment, Vol. 1 No. 1, 2011, pp. 6-13. doi: 10.5923/j.env.20110101.02.
Article Outline
1. Introduction
- In India most of the industries are situated along the river banks for easy availability of water and also disposal of the wastes. These wastes often contain a wide range of contaminants such as petroleum hydrocarbons, chlorinated hydrocarbons and heavy metals, various acids, alkalis, dyes and other chemicals which greatly change the pH of water. The waste also includes detergents that create a mass of white foam in the river waters. All these chemicals are quite harmful or even fatally toxic to fish[1,2,3] and other aquatic populations[4]. It is found that one-third of the total water pollution in India comes in the form of industrial effluent discharge, solid wastes and other hazardous wastes. Most of these defaulting industries are petrochemical industries, sugar mills, distilleries, leather processing industries, paper mill, agrochemicals and pesticides manufacturing industries and pharmaceutical industries. For such industries the surface water is the main source for waste disposal. Untreated or allegedly treated effluents have increase the level of surface water pollution up to 20 times the safe level in 22 critically polluted areas of the country. It is found that almost industry or the other[5-7]. Although all industries in India all rivers are polluted in most of the stretches by some function under the strict guidelines of the Central Pollution Control Board (CPCB) but still the environmental situation is far from satisfactory. Different norms and guidelines are given for all the industries depending upon their pollution potentials. Most major industries have treatment facilities for industrial effluents. But this is not the case with small scale industries, which cannot afford enormous investments in pollution control equipment as their profit margin is very slender. As a result there are sufficient evidences available related with the mismanagement of industrial wastes[8-15]. Consequently, at the end of each time period the pollution problem takes menacing concern. Some of the previous studies in the Arabian Sea have shown that the petroleum hydrocarbons ranged from 1.8 to 11.1mg/L in water, 1.84 to 5.81mg/g dry wt in sediments and 0.33 to 3.67mg/g wet wt in fish[16], while the total DDT in zooplankton samples in the Arabian Sea[17] varied from 0.083 to 0.563mg/L. The problem of water pollution has become still worse due to toxic heavy metals. The increasing trend in concentration of heavy metals in the aquatic environment has attracted considerable attention amongst ecologists globally during the last decades and has also begun to cause concern in most of the major industrialized cities. Untreated or allegedly treated industrial effluents often contains variable amounts of heavy metals such as arsenic, lead, nickel, cadmium,copper, mercury, zinc and chromium[12-15,18-20], which have the potential to contaminate crops growing under such irrigation. These heavy metals have a marked effect on the aquatic flora and fauna which through bio-magnification enter the food chain and ultimately affect the human beings as well. Heavy metal pollution is an ever increasing problem of aquatic bodies. In India, incidence of toxic heavy metal accumulation in fish, oysters, sediments and other components of aquatic ecosystems have been reported regularly[1,2]. These toxic heavy metals entering in aquatic environment are adsorbed onto particulate matter, although they can form free metal ions and soluble complexes that are available for uptake by biological organisms[21]. The metals associated with particulate material are also available for biological uptake[22], and are deposited in estuarine sediments[23]. Once deposited, binding by sulfides and/or iron hydroxides immobilizes trace metals until a change in redox or pH occurs[24,25]. Thus, surfical sediments, particularly the fine fraction, accumulate trace metals and provide a means for evaluating the long term accumulation of contaminants[26,27]. Hence there is a need for extensive monitoring of river water pollution along the industrial zones over long periods of time in order to describe average metal precipitation[28] and its trend, which is an essential component of any pollution control management. The day by day increasing tremendous pollution load along most of the rivers in India has prompted us to carry the systematic and detail study of water pollution in Kasardi River of Taloja industrial area of Mumbai.
2. Materials and Methods
2.1. Area of Study
- The Kasardi River which is the study area of present investigation receives heavy pollution load from nearby Taloja industrial area, which is one of the most rapidly developing and heavily polluted industrial belts of Mumbai. The industrial area is spread over 863.18 hectares of land consisting of about 600 large and medium scale industries like engineering units, steel processing industries, chemical units, paints, pharmaceutical units, textile industries etc. The study area lies between latitude 19°3'39"N longitudes 73°6'57"E. The main water source for the industrial consumption is Maharashtra Industrial Development Corporation (MIDC). The industrial area utilizes about 45,000 m3/day of fresh water. The effluent discharge, treated and untreated amounts to 28,750m3/day i.e., 64% of the total industrial effluents. Except for a few major industries, the medium and the small scale industries discharge their treated or untreated effluents through the unlined surface drains into the Kasardi River. Also atmospheric fallout from the chimneys and vehicle exhausts reach the river after washout. All this, has resulted in heavy pollution of the Kasardi River, thereby creating health hazards for local population and also disturbing the aquatic life of the river[7].
2.2. Climatic Conditions
- The weather of the study area is typical coastal sultry and humid. The average rainfall records from 1,500mm to 2,000mm. The place experiences the onset of the monsoon in the month of June and experiences monsoon till the end of September. The average temperature recorded varies from 25 to 42 degrees.
2.3. Requirements
- All the glassware, casserole and other pipettes were first cleaned with tape water thoroughly and finally with de-ionized distilled water. The pipettes and burette were rinsed with solution before final use. The chemicals and reagent were used for analysis were of analytical reagent grade. The procedure for calculating the different parameters were conducted in the laboratory.
2.4. Water Sampling and Preservation
- The water samples were collected randomly twice in a month in morning, afternoon and evening from upstream and downstream stations of the river. Polythene bottles of 2.5L and 2.0L were used to collect the grab water samples (number of samples collected, n = 19). The bottles were thoroughly cleaned with hydrochloric acid, washed with tape water to render free of acid, washed with distilled water twice, again rinsed with the water sample to be collected and then filled up the bottle with the sample leaving only a small air gap at the top. The sample bottles were stoppard and sealed with paraffin wax. The samples were collected every alternate month for the period of two assessment years 1999 and 2000. Water samples (500mL) were filtered using Whatman No. 41 (0.45μm pore size) filter paper for estimation of dissolved metal content. Filtrate (500mL) was preserved with 2mL nitric acid to prevent the precipitation of metals. The samples were concentrated on a water bath depending on the suspected level of the metals[29].
2.5. Heavy Metal Analysis by AAS Technique
- The analysis for the majority of the trace metals like chromium (Cr), cadmium (Cd), nickel (Ni), zinc (Zn), copper (Cu), lead (Pb) and iron (Fe) was done by Perkin Elmer ASS-280 Flame Atomic Absorption Spectrophotometer. The calibration curves were prepared separately for all the metals by running different concentrations of standard solutions. A reagent blank sample was taken through the method, analyzed and subtracted from the samples to correct for reagent impurities and other sources of errors from the environment. Average values of three replicates were taken for each determination.
2.6. Physico-Chemical Study
- The samples were collected were analyzed for temperature, pH, total dissolved and suspended solids,chloride content, oil /grease, BOD and COD values. The techniques and methods followed for collection, preservation, analysis and interpretation are those given by Rainwater and Thatcher[30], Brown et al.[31], ICMR[32], Hem[33] and American Public Health Association (APHA)[34].
3. Results and Discussion
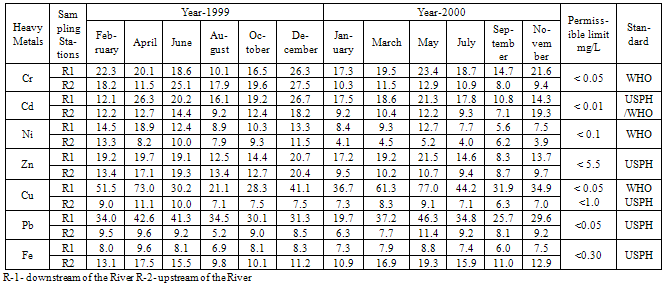
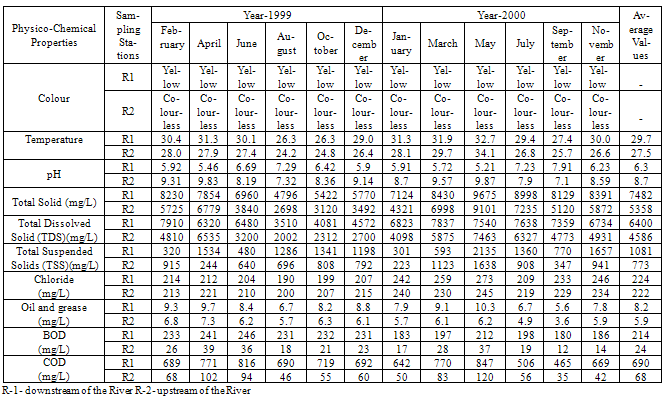
- The experimental data on heavy metal content and physico-chemical properties of water samples collected from Kasardi River flowing along Taloja industrial estate of Mumbai is presented in Tables 1 and 2.A number of elements are normally present in relatively low concentrations, usually less than a few mg/L, in conventional irrigation waters and are called trace elements. Heavy metals are a special group of trace elements which have been shown to create definite health hazards when taken up by plants. Under this group are included, Cr, Cd, Ni, Zn, Cu, Pb and Fe. These are called heavy metals because in their metallic form, their densities are greater than 4g/cc.
|
|
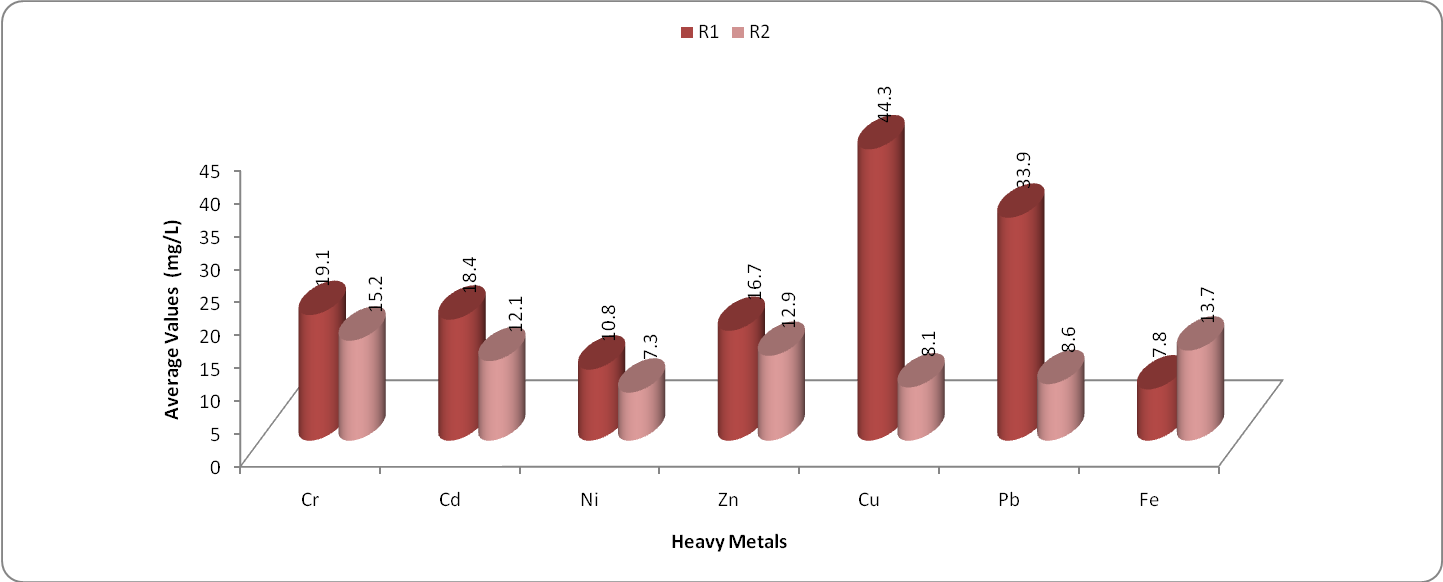 | Figure 1. Variation in average concentration of toxic heavy metals in water samples of Kasardi River of Mumbai |
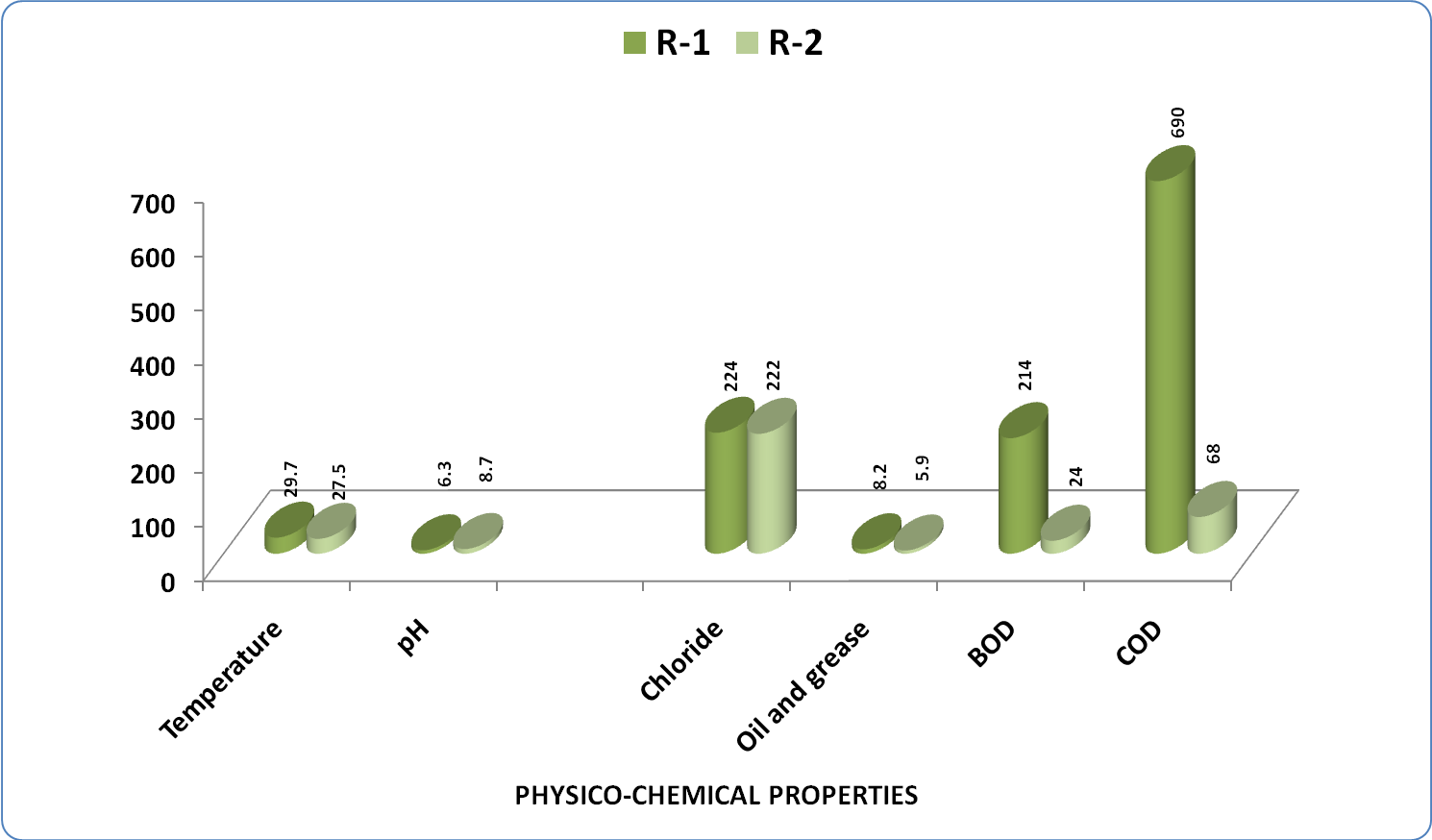 | Figure 2. Variation in average Physico-Chemical parameters in water samples of Kasardi River of Mumbai |
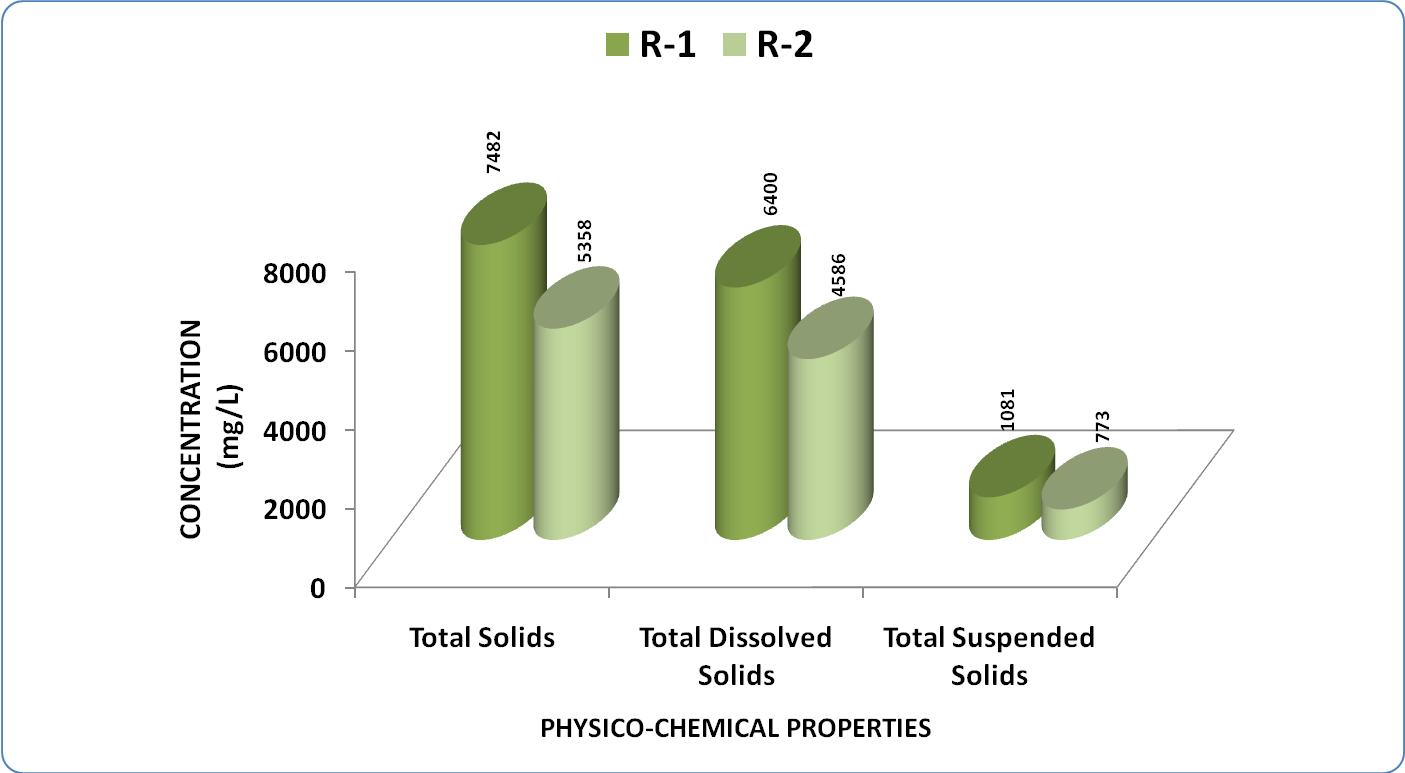 | Figure 3. Variation in average concentration of solid content in water samples of Kasardi River of Mumbai |
4. Conclusions
- Around the world as countries are struggling to arrive at an effective regulatory regime to control the discharge of industrial effluents into their ecosystems, Indian economy holds a double edged sword of economic growth and ecosystem collapse. The present experimental data indicates high level of pollution along Kasardi River of Taloja Industrial estate of Mumbai, India. The experimental data suggests a need to implement common objectives, compatible policies and programmes for improvement in the industrial waste water treatment methods. It also suggests a need of consistent, internationally recognized data driven strategy to assess the quality of waste water effluent and generation of international standards for evaluation of contamination levels. The existing situation if mishandled can cause irreparable ecological harm in the long-term well masked by short term economic prosperity.
ACKNOWLEDGEMENTS
- The authors are extremely thankful to SAP productions for developing and maintaining the manuscript template.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML