-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Education
p-ISSN: 2162-9463 e-ISSN: 2162-8467
2018; 8(3): 37-41
doi:10.5923/j.edu.20180803.01

The Effect of Molecular Model Sets on Students’ Academic Performance in Naming Organic Compounds
Harriet Gyasi1, Emmanuel Ofosu Ofoe2, Victus Bobonkey Samlafo3
1Effiduase Senior High School, Effiduase, Ghana
2Blessed Tutorial College, Kasoa, Ghana
3Department of Chemistry Education, University of Education, Winneba, Winneba, Ghana
Correspondence to: Victus Bobonkey Samlafo, Department of Chemistry Education, University of Education, Winneba, Winneba, Ghana.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study sought to investigate the effect of molecular model sets on naming simple organic compounds with reference to the International Union of Pure and Applied Chemistry (IUPAC) system of naming. A quasi-experimental design using molecular model sets were used on students in form three science classes at Effiduase Senior High School in the Sekyere East District of the Ashanti Region of Ghana. A control group was taught using the traditional approach whilst the experimental group was treated using the molecular model sets. Analysis of the pre and post-tests scores showed more improvement in the performance of the experimental group than the control group. A paired sample t-test, on responses of students’ attitudes, showed a statistically significant difference between the mean scores for the experimental group before and after the treatment. The study revealed that the integration of molecular model set approaches to teaching could help reduce, if not remove completely, the difficulties students face in naming organic compounds according to the IUPAC nomenclature.
Keywords: Molecular model set, Scientific model, Senior high school, Model, Organic chemistry
Cite this paper: Harriet Gyasi, Emmanuel Ofosu Ofoe, Victus Bobonkey Samlafo, The Effect of Molecular Model Sets on Students’ Academic Performance in Naming Organic Compounds, Education, Vol. 8 No. 3, 2018, pp. 37-41. doi: 10.5923/j.edu.20180803.01.
Article Outline
1. Introduction
- In Ghana, the West African Examination Council (WAEC) is the body responsible for conducting the Senior High School final examinations. The WAEC Chemistry Chief Examiner’s Report has continuously commented on the poor performance of most students in using the International Union of Pure and Applied Chemistry (IUPAC) nomenclature to name organic compounds. According to 2002, 2004 and 2006 WAEC Chief Examiners’ reports, candidates showed weaknesses in IUPAC naming of simple organic compounds [1, 2, 3].Organic chemistry is difficult from many students’ point of view, especially when looking at structures from a three-dimensional viewpoint. There is, therefore, the need to adopt effective teaching strategies, integrated with appropriate teaching aids. Hence, this study used molecular model set as an appropriate teaching aid in the naming of organic compounds to improve students' conceptual understanding and academic performance in the use of IUPAC system to name organic compounds. The purpose of using molecular model sets as teaching and learning materials fits into the ideas of constructivism. It excites and maintains the interest of students, ensures practical work, enables learners to acquire skills and ensures acquisition of first-hand information. As students learn by building and naming various molecules, they become actively engaged in experience-based learning, which is one of the keys to the construction of new knowledge [4].In spite of the benefits associated with the use of molecular models sets to promote students’ conceptual understanding of abstract chemistry concepts, experience shows that teachers seem not to have ample time to utilise these models to remediate chemistry students’ understanding of naming of organic compounds. For this reason, this study was designed to see if it could be used as a remedial instruction to weak and average students and also for the general improvement of performance of students. Hence Effiduase Senior High School students were chosen for this study.
2. The Theoretical and Conceptual Framework
- Chemistry as a science and as a school subject is based on models [5]. Chemistry occupies a special place in science since few of the macroscopic observations cannot be understood without recourse to sub-microscopic representations or models. Accordingly, the learning of chemistry necessitates the development of chemical models as well as an understanding of their use in specific contexts. This is a process that is a challenge to both teachers and students since it is multifaceted, and often time-consuming.The chemical models in focus here can be generally categorised into three different groups. These different groups, which are differentiated through their intent and use, are referred to as scientific models, educational models and students’ expressed models [5].Scientific models are used for describing and presenting scientific findings and are intended for the scientific community. These scientific models’ development by scientists and their use change progressively over time. The succession of models used for explanations and predictions can be seen even over relatively short time spans. A classic historical example of this progression can be found in the development of models of the atom presented by J. J. Thompson and Rutherford in 1904 and 1911 respectively.Educational models comprised a manifold of models under the following headings: curricula models, consensus target models and teaching models all intended for educational purposes. These models are altered through interpretation. Curriculum developers interpret scientific models and transform them into school science curricula, i.e. curricula models [5]. Textbook authors then interpret the curricula models and these are subsequently transformed into consensus target models designed for different educational levels. These consensus target models are commonly used for learners at different levels of education and can be deduced from textbooks. Examples of consensus target models from chemistry at this educational level (Senior High School) are; the general composition of an atom and its subatomic particles or symbolic representations of the atom. Students’ expressed models are intended for students to learn the formally introduced teaching models and their respective frameworks so that, they become of use when forming explanations. The learning of teaching models can be seen as yet another interpretation. Students’ own interpretations of teaching models, presented both visually and verbally, are referred to as students expressed models, which represents the third category of models [5]. This has led to the construction of molecular model sets in teaching structures and spatial orientations of organic molecules or compounds in space.Teachers subsequently interpret the target models and transform them into teaching models. Teaching models can involve yet further simplifications such as teaching analogies that are illustrations of an idea, object, event, processes or system. Teaching models can utilise phenomena from the macroscopic to sub-microscopic levels interrelated into frameworks, i.e. a web of ideas within a particular scientific subject [5]. In organic chemistry, students find it difficult to connect the molecular structure, its geometry and molecular characteristics of a compound in order to appreciate and understand the reactivity of the compounds [6]. A study conducted in Nigeria, a member country of WAEC indicated that IUPAC nomenclature of organic compounds is one of the difficult topics in the Senior Secondary School syllabus [7]. Students’ problem with IUPAC nomenclature has also been reported elsewhere in the world. Earlier studies conducted revealed that students have difficulties in the use of IUPAC nomenclature [8]. A similar study conducted in Ireland revealed that second level Irish pupils have difficulty in naming and writing of formulae of organic compounds [9]. A diagnostic test conducted to assess students’ knowledge in organic chemistry confirmed the WAEC chief examiners’ reports and other research findings. Apart from students’ difficulty in naming organic compounds, the lack of teaching and learning materials worsen the already bad situation in students’ performance.Understanding the particulate nature of matter and the interpretation of the symbols and visualizing the spatial orientation of atoms in molecules are essential skills students need to acquire in solving problems in chemistry in general and organic chemistry in particular.To help students to perform well in chemistry, teachers should develop new approaches to teaching it, such as adapting teaching strategies based on the conceptual change model, presenting the historical change of a theory, using concrete models, and using technological tools. For instance, multimedia tools, which integrate the animation of molecular models, video clips of chemical equilibrium, or real-time graphics, provide students with varied opportunities to visualize chemical processes at the microscopic level. While empirical studies assert the value of using teaching models and technological tools for learning chemistry, however, little is understood about how teaching models actually support students’ learning. Moreover, how students use these teaching models evolve over time in classroom settings, and what features of a technological tool help students to develop conceptual understanding of chemical representations [10]. In a study which investigated students’ mentality in relation to their attitude concluded that students' ways of thinking about learning are strongly correlated with factors such as personal involvement, purpose, and personal achievement. Also, the study showed that the purpose and personal achievement factors were strongly correlated with their ways of thinking about learning [11].In another objective in the same study to establish the factors that determine the students' ways of thinking about learning and school performance, the statistical results obtained showed that there was a significant interrelationship between attitude and performance. Students, who have positive ways of thinking about learning and get involved in activities, achieve higher academic performance than those who make minimal efforts in this regard. Thus, students with good results believe that learning provides satisfaction, and just being a student is not enough to feel fulfilled. These students are willing to take on additional tasks and condemn superficiality in school tasks. Students with higher scores set specific goals for learning aiming at acquiring new knowledge, not just completing a routine activity, and strive for excellent school performance. Also, students with higher performance consider learning a way towards personal development which translates into success in life [11].Research questionsThe study was guided by the following research questions:1. What is the effect of molecular models sets on students’ academic performance in naming organic compounds?2. What effect does the use of molecular model sets have on the attitudes of students in the naming of organic compounds using the IUPAC nomenclature?
3. Methodology
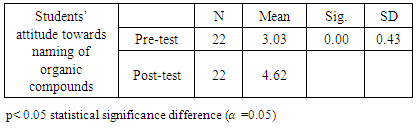
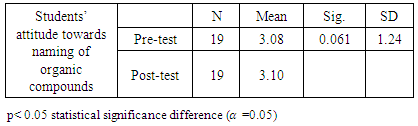
- Research Design A quasi-experimental design was used to gather data for this study. A pre-test administered to measure the performance of the students with regard to their previous knowledge on the topic was used to categorise the students into two groups. Students who were below the pass mark were put in group B, as the experimental group, and those above the pass mark were put in group A, as the control group. The experimental group was taken through a treatment session for six weeks, where they were exposed to construction and naming of different isomers of aliphatic hydrocarbon using molecular model sets. The control group was taught using the traditional method of teaching within the same period.Population and sampleThe target population for the study comprised all science students in Effiduase Senior High School (SHS) in the Ashanti Region of Ghana. However, the accessible population consisted of eighty-two form 3 SHS students of Effiduase Senior High School as the topic is indexed in the third year syllabus. In other words, it is in form three that SHS students are taught organic chemistry, hence form three students formed the actual population. Two form three science classes were purposively selected for the study. A pre-test was used to put the two classes into two groups, a control group and an experimental group. A total of forty-one students took part in the pre-test which formed the sample size. Nineteen of these students scored above the pass mark and were made the control group. Twenty-two students’ scores were below the pass mark and they formed the experimental group.Research InstrumentsTests (pre- and post-test) and questionnaire were the main instruments used to collect data for the study. The post-test items (30 questions) were developed and trial- tested with form three students in another school to ensure internal consistency of the test items. The items included twenty items on the naming of organic compounds and ten items on writing condensed formulae, and structural formulae (isomerism). It was marked and scored for 50 marks. The reliability index of the instrument was 0.67 using Cronbach’s alpha reliability test. The Cronbach’s alpha of 0.67 is approximately 0.7 and this was acceptable [12]. The Students' Attitudes Questionnaire (SAQ) used for this study was adapted and modified to suit the study [13]. The questionnaire was categorized into Pre- and Post-tests items and consisted of 7 items with a 5 point Likert scale, using SD-strongly disagree, D-disagree, NS- Not sure, A-agree, SA- strongly agree. Data Collection ProcedureThe pre-test was also used to engage the students to know the types of preconceptions that students brought to the classrooms. This was done for both the control group and the experimental group.After the pre-test, the control group was taught using the traditional method of teaching whilst the experimental group was treated using the molecular model sets. The students were taught for six weeks in the naming of alkanes, alkenes, alkynes and the construction of their respective isomers. In the first three weeks, the students were taught alkanes, the remaining weeks were divided into two for alkenes and alkynes.The control group, who were taught through the traditional method, were introduced to the guiding principles in naming alkanes using the blackboard and chalk method, whilst students listen. No visual representation aids were used. The teacher simply drew 2-dimensional (planar) structures, used the guiding principles to name them. When the students understood the application of the guiding principles in naming the structures, the teacher drew structures for the students to name. Next, the teacher gave names of structures and used the guiding principles to draw them. The students were given names of structures and asked to do same. The students were asked to explore other structures and name them. The same procedure was followed in naming and drawing structures of alkenes, alkynes and their respective isomers.The experimental group were also taken through the same procedure of teaching the control group in addition to visual representation aids, the molecular model sets. For example, the teacher simply drew 2-dimensional (planar) structure, used the guiding principles to name it on the blackboard. The molecular model set was then used to construct a 3-dimensional structure of it to enable the students to get a clear geometric representation of the structure. The teacher gave a list of structures for students to construct and name. The students were paired and encouraged to use the molecular model sets provided to construct structures and their isomers given to them.At the end of the teaching period, a post-test was administered to both groups to measure their performance. The answer sheets were collected after 60 minutes and were scored after which the marks were recorded. The purpose of the post-test was to evaluate the achievements of the two groups in using the IUPAC system in the naming of the organic compounds. The administration of the questionnaire was done for the two groups. The same questionnaire was given to the students to evaluate the lessons. Data AnalysisDescriptive and inferential statistics were used to analyse the data. Responses on students’ attitudes towards the systematic naming of organic compounds were analysed using a pair sample t-test. The paired sample t-test was used to find out whether or not there was a statistically significant difference between the mean scores of the control and experimental groups at the statistically significant level of 0.05, after using the two methods of teaching.
4. Results and Discussions
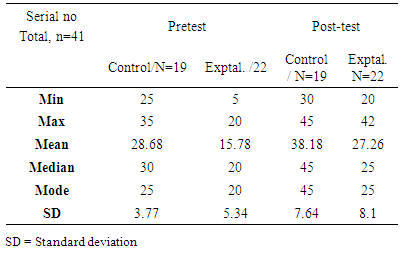
- Analysis of Research Question One (RQI)RQ1: What is the effect of molecular models sets on students’ academic performance in naming organic compounds?From Table 1, the marks obtained by control group (students) in the pre-test ranged from a minimum of 25 to a maximum of 35 with a mean of 28.68 (SD= 3.77), whilst the experimental group scores ranged from a minimum of 5 to a maximum of 20 with a mean of 15.78 (SD= 5.34). About 46.3% representing 19 students failed the pre-test. However, in the post-test, the scores of the control group ranged from a minimum of 30 to a maximum of 45 with a mean of 38.18 (SD= 7.64) whilst the experimental group ranged from a minimum of 20 to a maximum of 42 with a mean of 27.63 (SD= 8.1). Only 12.20% representing 5 students obtained scores which were below the pass mark of 25. With the use of molecular model sets, even very weak students scored 20 out of 50 marks. This is a drastic positive change in performance. This closed the gap between the two groups in terms of performance. This is evidenced by Pearson correlation (0.13) which does not show any correlation between the post-test scores of the control and the experimental group. As the correlation coefficient value approaches zero (0), the relationship between the two variables becomes weaker.This implied that molecular model sets had a positive influence on the academic performance of the students in the experimental group. The use of scientific models in science education has been shown by many researchers to be beneficial to students’ understanding of chemical concepts [14, 15]. However, With respect to research question one, the SHS students in the experimental group exhibited a higher gain in marks than their counterparts in the control group in the post-test (Table 1). The experimental group, on the average, gained 11.8 marks (27.26-15.78) and that of the control group was 9.5 marks (38.18-28.68) when their pre-test and post-test marks were compared. The higher performance by students in the experimental group might be as a result of the use of the molecular model sets. The result of the study is supported by other studies [16, 17]. Teachers in the control group used traditional teaching approach, limiting students’ experience to be partially or completely inadequate. This result is in accordance with the findings of an earlier study [17].
|
|
|
5. Conclusions
- The study revealed that students introduced to molecular model sets teaching strategy, developed positive attitudes towards the naming and construction of isomers of organic compounds. This is as a result of the practical nature of the teaching approach. Furthermore, the study indicated that the integration of molecular model sets in teaching organic chemistry topics enhanced the students’ conceptual understanding. It can also be deduced from the study that, when the appropriate teaching and learning materials (TLMs) and methods were used, the learners’ attitude improved.Teachers are therefore advised to use varied methods of teaching that would satisfy the students’ individual abilities. The Curriculum Research Development Division (CRDD) should ensure that the syllabus is not loaded so that teachers would not concentrate on the theory aspect alone but would also include a lot of practical lessons. The Ghana Education Service (GES) should also make adequate provisions for training programmes such as seminars and other workshops for science teachers so that they can effectively vary their teaching methods to improve chemistry teaching and learning in the Senior High Schools.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML