-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Education
p-ISSN: 2162-9463 e-ISSN: 2162-8467
2018; 8(2): 32-36
doi:10.5923/j.edu.20180802.03

Conceptual Reorganization of Phenomena Involved in the Transformation of Matter during Higher Education
Luana Ehle Joras1, Blessing Ariyo Afolabi2, 3, João Batista Teixeira da Rocha3
1Postgraduate Program in Sciences, Chemistry of Life and Health, Federal University of Santa Maria (UFSM), Santa Maria, RS, Brazil
2Department of Biochemistry, Bowen University, Bowen, Osun State, Nigeria
3Department of Biochemistry and Molecular Biology, Federal University of Santa Maria (UFSM), Santa Maria, RS, Brazil
Correspondence to: Luana Ehle Joras, Postgraduate Program in Sciences, Chemistry of Life and Health, Federal University of Santa Maria (UFSM), Santa Maria, RS, Brazil.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study involved the understanding of 26 students (15 undergraduate and 11 postgraduate students) in the conceptual change on the transformations of physical and chemical matter through the identification of 19 physical and chemical phenomena that occur in people's everyday lives. The information was collected based on the Free Categorization Task (FCT) [1]. The results show how the students organize their conceptual knowledge according to their levels of education. Thus, it is possible to verify that the increase in the level of education collaborates positively with the understanding and identification of the phenomena involved in the process.
Keywords: Phenomena, Conceptual change, Level of education
Cite this paper: Luana Ehle Joras, Blessing Ariyo Afolabi, João Batista Teixeira da Rocha, Conceptual Reorganization of Phenomena Involved in the Transformation of Matter during Higher Education, Education, Vol. 8 No. 2, 2018, pp. 32-36. doi: 10.5923/j.edu.20180802.03.
1. Introduction
- Literacy in science education is important for active and informed citizenship in relation to the world and its events. Science education contributes to the process of scientific literacy, as it stimulates students to construct meanings and expand knowledge about the world, enabling them with conditions to amplify culture and perceptions about the use of science and technology. However, in several developing countries, students' performance is very poor, particularly in Brazil, which is among the worst countries in the Programme for International Student Assessment (PISA) ranking in the areas of science, mathematics and reading [2]. The reasons for the low level of literacy in science education and other subjects in Brazil can be attributed to several factors, including the hegemony of traditional approaches to teaching, where the old bureaucratic system of teaching and learning is used. In this regard, Santome [3] encourages teachers to work in an innovative way, where questions arise naturally without imposing them, as it ensures that an inclusive work plan should be free and thought-provoking. The bureaucratic system prioritizes routine learning and the use of low cognitive skills, where teachers have no clear ideas about their students' actual understanding of the subject matter being taught. According to Morin [4], it is necessary to contextualize the subject that will be taught, as fragmented pieces of information will not be accommodated in the cognitive structure of the learners [5].In addition to the low level of investment in education, abstract and excessive content of the schools curricula also contribute to the low performance of young students in science education [6-9]. Unfortunately, the problem in basic schools is the same found in most Brazilian universities. Consequently, we can expect pre-service teachers in science courses to be poorly prepared to innovate in their teaching methodologies.The literature has pointed out difficulties in providing a suitable course of chemistry that covers the basic chemistry concepts, for instance, phenomena occurring in the matter surrounding us and definitions of chemical reaction are not easily understood by students [7-13]. Here we have done a study based on the study by Stavridou and Solomonidou [1] on "Conceptual Reorganization and Construction of the Concept of Chemical Reaction during Secondary Education", to evaluate the conceptions of pre-service chemistry teachers and postgraduate students in biochemistry and science education about change in matter. In particular, we sought to determine whether conceptions improved or not with the level of education. The conceptual understanding of matter change can be seen as a complex phenomenon. It encompasses an understanding of unique concepts at the atomic level or more complex concepts such as redox reactions and molecular rearrangements [11-13]. In this sense, authors affirm that the distinction between the macroscopic, microscopic and sub-microscopic levels transcends what teachers and textbooks mention [12, 13]. One of the major difficulties in the process of acquiring scientific knowledge by the students is that spontaneous conceptions tend to be resistant to changes [12]. In science education, the models of teaching aiming to stimulate conceptual changes are recent. The teachers' understanding of students' preconceptions or spontaneous conceptions are crucial to facilitate the conceptual changes. Knowledge-related changes can arise through conceptual networks and semantic categories. The semantic categories are accompanied by a set of properties that determine specific categories of a given element. Thus, the semantic categories are adequate to represent conceptual changes, for example, referring to the concepts of transformations of matter by physical and chemical phenomena [14].A more constructive view of learning considers that we should understand the students' perspective, before and after the proposed activities. The scientific view arises according to the subjects the students need to know and this should be emphasized. The desire to stay in school is associated with the pleasure of the students to learn when they perceive that the subjects can be related to their daily lives [15, 16]. For instance, undergraduate students taught using traditional approaches, for instance, practical activities of the type “follow the cookbook recipes”, will have minimal understanding of the contents. This in-turn criticizes the form of the teaching and not the students being taught [8].In Piaget's constructivist perspective, human knowledge is constructed during the interaction of apprentices with the environment. In effect, the knowledge is the balance between assimilation and accommodation of schemes resulting from the interaction between the individuals and physical objects in the world [5, 17]. In respect to this, many criticisms arise against the traditional teaching, where the student receives information passively without mentioning the previous knowledge acquired during his life, nor whether the new knowledge has been assimilated and accommodated [5, 17].According to Nunes and Adorni [18], students often fail to learn chemistry because they cannot make a connection between classroom content and everyday life and thus become uninterested in the subject. In addition, chemical models can be very complex and abstract for adolescents [13, 20]. According to Lopes [20], the adaptation of the scientific knowledge to the students’ language is a difficult process of transformation of the complex scientific knowledge into more comprehensible set of information for students. The simplification of the knowledge to be taught (scholarly knowledge) has to follow some rules of adaptation and transformation to make it appropriate as learning objects (didactic transposition), without losing its scientific essence. In fact, the use of didactic transposition is a challenge for teachers and the schools accustomed with the bureaucratic system of teaching. Consequently, it is important to reflect not only on the attributes of the knowledge themselves but also the features of the students, their previous knowledge and/or ability for reasoning [21]. For different authors [13, 22], conceptual understanding in chemistry involves the ability to solve problems using three levels of understanding: macroscopically (observable), molecular or microscopically (particle level) and symbolically. Macroscopically, it covers models of the world based on knowledge about observable chemical phenomena. At the molecular level, the focus is on knowledge based on imagination (e. g what happens to atoms and molecules during physical and chemical changes?). Finally, symbolically, it facilitates explanations of chemical phenomena represented in different ways (for example, mathematically, verbally or in chemical models). Thus, understanding of chemistry involves the ability to reflect macroscopically, molecularly/sub-microscopically and symbolically [13]. Trevisan and Martins [6], reinforce the need to discuss chemical education, prioritizing the contextualisation of contents with students' daily life, allowing the understanding of several issues, such as the disappearance of substances, impacts of the chemical industry on the environment and the production of waste by modern society, among others. Generally, the way in which the content is taught determines the low motivation of the students toward chemistry, since most of the content is viewed as abstract and difficult to comprehend [23]. Contextualization has an important role in teaching-learning because it links knowledge to its origin and application and in addition, stimulates the creativity, imagination, and curiosity of the student [24]. Considering the importance of an in-depth understanding of the basic aspects of the transformation of matter by the future teachers of basic education, the objective of this work was to investigate the conceptual change of students in different levels of university education, using simple questions about some examples of matter transformation that are present in our daily lives.
2. Methodology
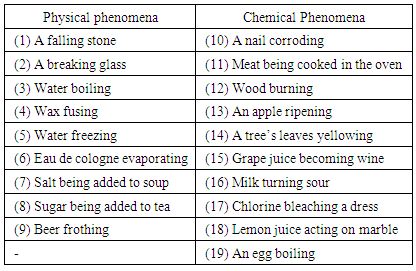
- Effective science education teaching can facilitate the understanding the phenomena that occur in everyday life from the point of view of chemistry and physics. In order to detect the semantic categories of students and their evolution, the Free Categorization Task (FCT) based on Stavridou and Solomonidou [1] was used. According to the FCT method, students can freely categorize a diversity of daily physical and chemical phenomena according to their own conceptions. The present study was carried out with the participation of 15 undergraduate students of Chemistry education Course (7th and 8th semester) and 11 postgraduate students of Biological Sciences: Biochemical Toxicology and Sciences Education: Chemistry of Life and Health. These two groups of students of different levels of education provide an idea of the possible conceptual evolution along the levels of education in the university (undergraduate and post-graduate). In the table below, 19 daily phenomena are arranged (Table 1) [1, 25]. Of these, nine phenomena are identified as physical and ten as chemical [1, 25].
|
3. Results and Discussion
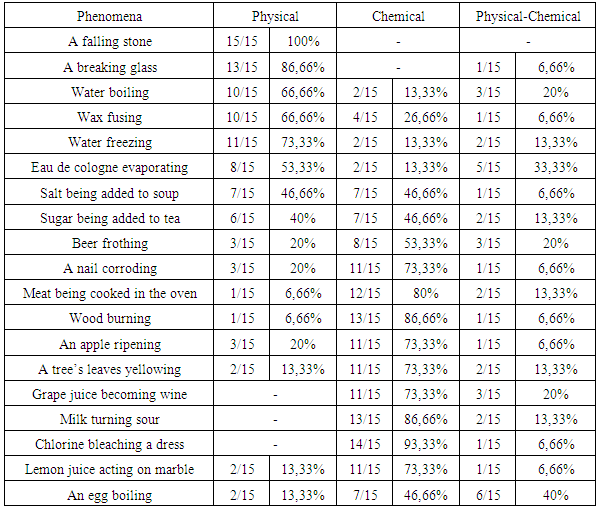
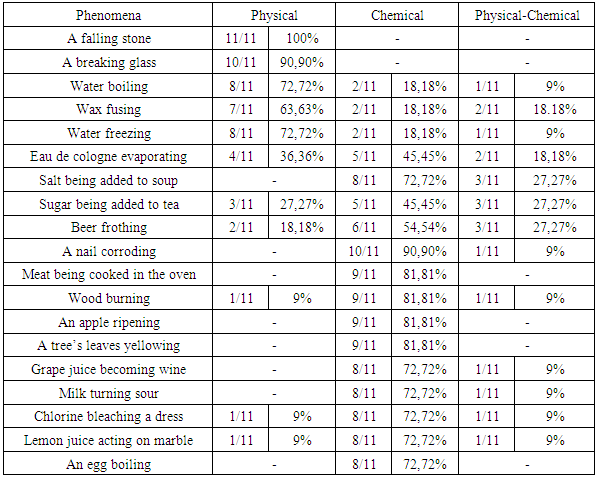
- This study investigated the understandings and conceptions of students in different levels of higher education on questions related to the study of sciences. The results presented in Table 2 and 3 demonstrated that the conceptions of post-graduate students were closer to the scientific knowledge than that of pre-service chemistry teachers.
|
|
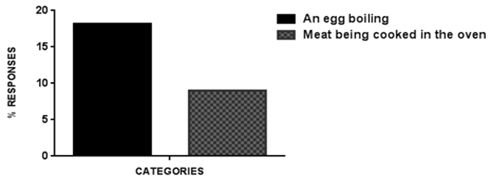 | Figure 1. Biological phenomena |
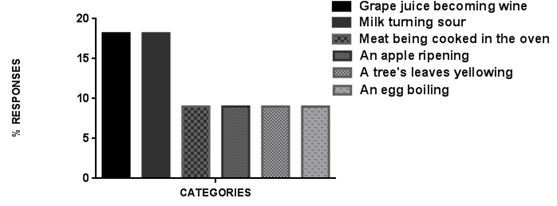 | Figure 2. Chemical-biological phenomena |
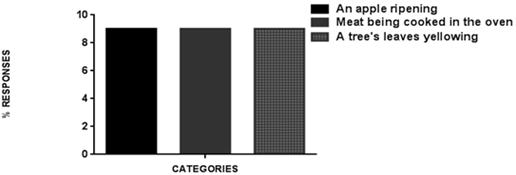 | Figure 3. Physical-chemical-biological phenomena |
4. Conclusions
- The conceptual change of students should be emphasized by science teachers and researchers in order to improve the students' conceptions. In this way, it is important to develop more effective pedagogical approaches to facilitate the understanding of daily life phenomena at the macroscopic and microscopic level.It was noticed here that the number of correct identifications of the phenomena improved somewhat with the level of education of the students. Post-graduate students tended to better understand that physical and chemical phenomena can occur simultaneously and have proposed new categories to explain the phenomena. Despite of the tendency of post-graduate students to perform better than pre-service chemistry teachers, the levels of abstract reasoning of all the students were far from the expected for their educational levels. The main qualitative impression was that students did not explore the molecular or microscopic (and sub-microscopic or atomic and abstract levels) reasoning properly. Indeed, the results indicate that pre-service teachers and post-graduate students should be introduced to the triangulation approach [13] in order to better perceive and learn about the microscopic and symbolic aspects involved in the transformation of matter.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML