-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Education
p-ISSN: 2162-9463 e-ISSN: 2162-8467
2015; 5(6): 151-157
doi:10.5923/j.edu.20150506.01

Social and Cultural Aspects of Education, Learners Responsibilities and Teaching Method
Sohrab Abdollahi
Department of Chemistry, Payame Noor University, I. R. of Iran, Tehran, Iran
Correspondence to: Sohrab Abdollahi, Department of Chemistry, Payame Noor University, I. R. of Iran, Tehran, Iran.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

It seems that the advancement and evolution of technology in education have not brought expected results regarding upbringing genius and scientist comparing to the past. Abiding, perseverance, tenacity and tendency, are four essential factors by which educational challenge and durability are determined. In this new century, due to technology and huge changes of cultural-social aspects, we see contradictory relationship between educational facilities and learning process. It seems that the learners have lost partially or in some cases totally their power of abiding and perseverance. Economical and social facilities in both advanced and advancing countries and luxurious living have made young generation more apathetic rather than being more responsible. These days the formal teaching does not work properly. Combination of Socratic and formal method may rationalize the way that a solid learning and incitement can be achieved. Five or ten minutes of the beginning and end of the class can be allocated for Socratic method by asking question and debating among students and the rest of the class may be allocated for formal teaching. This mixed method can eliminate the shortcoming of Socratic or formal method and instead having the advantageous of both.
Keywords: Teaching method, Evaluation, Abide, Socratic method, Formal-Socratic
Cite this paper: Sohrab Abdollahi, Social and Cultural Aspects of Education, Learners Responsibilities and Teaching Method, Education, Vol. 5 No. 6, 2015, pp. 151-157. doi: 10.5923/j.edu.20150506.01.
Article Outline
1. Introduction
- There is a big question regarding education and potential of learning these days which are highly advanced in educational facilities such as computer, internet, communication systems and variety of educational software and hardware. In spite of all these educational facilities, why the learner productivity, excitement and perseverance have diminished compare to old days when the pens were a piece of charcoal and the papers were leathers or piece of tin plate [1-3]? How genius scientists such as great Newton, Maxwell and faraday were brought up in that times? There are many researches about the impact of media and technology in schools [4, 5]. Of course, we do not want to underestimate the effectiveness of media and technology in education [6] and at least they have enhanced quantity of learning because with this huge population of the world, we could not go simply by old fusion pen and paper. However, from quality point of view, regarding education, we have not achieved expectable level regarding real education. For hyperactive (ADHD) students it may even get worse and technology such as computer surely put these kinds of students in trouble. It needs very deep insight and rational studies to find out how come such a conflict some times may exist between technology and learning. Many different factors have affected this educational dilemma. Cultural, economical, social, technological and even political aspects have played a crucial role in behavior and cognitions of learners. How we can convert or relate these aforementioned factors to educational meanings and expressions? With more observation in behavioral spectra of learners we deduce that perseverance [7], obedience, tenacity [8] and apathy are the main educational related factors in this educational crisis. Still in some developing countries such as Iran, another factor is involved such as using education as an important source for merchandise and making money. Any time that education involves with merchandise then, learners and education are the main losers.In general, the most of the young generation these days have problems. These problems, directly or indirectly affects the educational behavior of these generations. This trend is almost unavoidable, because huge enterprises, largeness of cities, luxuries of livings and explosion of ideologies have important role in educational confusion and defects. Economical situation, specially in developing countries which have oil as a main resources of income, supports dealership, bourgeoisie activities, technician professions and in general income sources that do not need higher educations. Fame and money which are involved with widespread activities of sports and championship, being artists or singers are all different causes for learner apathy regarding learning and education.
2. Learning Problems
2.1. Abiding and tendency
- These two terms are separated key words in education. The term “abide” means that learner is responsible and committed to gain knowledge. He/she is responsible toward parents, society, world and God to stand strong against all learning hardship and abide by learning process. Abiding means domination to ignorance and laziness. It means forcibly to put some one behavior in a specific framework for learning. In fact, it needs energy and strong will to do that. Abiding in education means: You should do something that is beneficial for you and your country even if you do not like it.However, the term “tendency” is different from “abiding” term. Tendency comes with subjective potential and essential energy which had been existed in individual from the beginning. Therefore, it does not need too much will or mental energy to do what you like. The point is that for specific issue, just tendency alone is not enough and in addition to tendency, you should be able to abide until you implement a learning process. For instance: A morning John wakes up and he has great tendency to eat fresh pancakes for breakfast. He has to buy the stuff from grocery down street a couple of mile away. He does not have a car and therefore he is not in the mood and does not have required stamina to execute his desire. So, he decides to forget about it and not to do that. Here, in fact, you see that, even though, John has strong tendency to have fresh pancake for his breakfast, but since he does not abide to walk to the selling shop to get pancake, therefore he decides to forget about it. We can conclude that, for student, the hard step in learning process is abiding but not tendency, because majority of students, actually like to learn and educate themselves but it is hard to abide their wish.For today’s society, the real problem for education is lack of “abiding” by students. Students do not feel any responsibilities toward actions which are desirable for society and requested by that. They do not abide with learning for prosperity of the society [9-11]. For this feeling, different reasons have been presented:1. Diminishing of nonphysical disciplines, lack of respect and responsibility in childhood period.2. The non-practical interference of some inapt psychologist and counselors3. Childcracy 4. lack of warm hearted family 5. Presence of enormous entertainment (e.g. TV and video games).6. apathy
2.2. Evaluation and Education
- For education, evaluation is the most effective process to assess the results and has direct influence on culture, economy and in general prosperity of a country. Without evaluation, the development of a country will be gradually hindered. Evaluation and exams act such as filter for the separation of good educational path from the one that is not proper. Fortunately, these days, due to development of educational instruments and technologies, advancement in evaluation sciences, computer and statistical methods, we can differentiate good educational systems from week one. However, the bad news is that, the evaluation, most of the time is collapsed because of ignorance and misused by opportunist people and this is true especially in developing countries such as Iran. Therefore, excellences may be based on criteria which are not related to education. Experiences have shown that, a successful developed country has always a precise solid evaluation system. Good evaluation systems will provide potential managers, engineers, doctors, and governmental authorities for the positions and vacancies in the country. For the economical flow, we need different potential of human resources, and this can be achieved only by spectrum of abilities and talented persons from strong to weak educated people. Therefore, the high quality and sensitive jobs will be assigned to well educated people and low quality jobs will be assigned to poor educated individuals. In fact this differentiation in potentials will provide good economy, society and solid political strategies in a country. In some undeveloped or developing countries such as Iran, Pakistan and Afghanistan, Iraq, Syria and… due to anarchy and wrong criteria for development, education cam not have any role to provide potential people for right position in country. In fact they put grass on front of dogs and bones in front of donkeys to feed them. This is exactly what happens in Iran and the other aforementioned countries.
2.3. Educational Businesses
- In general, any time that education involves in commerce and profiteering, then, this is education that spoils. Socrates believes that education is sacred and should not be mixed with worldly issues and should not be sold. The education should be served and delivered to people free. Further that educated people in teaching should act like amateur and not knowledge seller. Unfortunately educational businesses are epidemic in some developing countries such as Iran (contrary to constitutional principles; acts 3 and 30).Because of luxurious community of these days, in fact, you can not have a simple life in the society. Every individuals (including teaches or University professors), are struggling hard to enhance their income so that be able to stand fairly in a good shape and make a desirable condition for their family and children. In Iran, this issue among our educational administrations is such strong that, unfortunately they may see no any reason or incitement for the real evaluation so they are trying to graduate more and more students to get more money. Many teachers may spend more time for out of job work to get more money. In this case, it is clear that the quality of education will decline. Consequently, sloppy education, demands sloppy evaluation and worthless examinations. The teachers should please students, parents and schools administrators. Therefore, by using sloppy evaluation, they can cover their weaknesses. The bad news is that, this kind of tragedy may gradually change to a common fact and seems natural to people after a while.A teacher who teach part time in other school or educational institution, in addition to his permanent school, he would not work conscientiously in his temporary job and on the other hand is too tired to work actively and precisely in his permanent school. So he will destroy his educational morality and validity in both jobs. This trend in ill society such as Iran, become epidemic and in fact this would be the end of educational oriented country. The detail reviews of Iran educational systems simply reveal this fact.As you see, the ways that teachers and universities acting for more income, can be a main factor to decrease the quality of education, consequently, weakening the evaluations and exams.In educational systems of Iran, both universities and school systems (ministry of higher education and board of high school education), unfortunately are involved in this kind of educational crisis.Other main key issue of this tragedy is some irrational governmental regulations which create and provide very low quality standards for entrance exam. Numerous establishments of nonsensical institutions so called universities of Payme Noor and Azad, without any standard and acceptable criteria, extending the number of graduate students toward masters and doctorate degrees based on non-educational standards.
3. Teaching Methods
3.1. Formal Method
- Today, educational systems in the world are based on formal process and teaching is totally organized and perform under vastest procedures, guidelines, predetermine steps and controlled learning sources and evaluations.In the formal teaching method, the teacher hands over some notes and teaches under some predetermined programs which are assigned to specific class. The teachers transfer the knowledge by giving organized lectures. In this method, teacher is the only one in control of the class, students and a person who talks. The students should listen and if possible raise questions during or at the end of the lecture. The teacher, usually hands over a note, gives a lecture or refers some books and some times provide students with a copy of needed materials, and some other teachers may do all.The formal teaching method has some advantageous. Learning process is faster and needs less time and neat materials are readily presented via notes or lectures. This method is more systematic. However, the formal method can be effective if both teachers and students accept required responsibilities. It means that the teacher should up to date his knowledge and learner follow carefully the teacher’s instruction and read the notes and books. In addition, the students should refer to more books and not just content themselves with teacher’s books, lectures and notes and try to search for more knowledge.The disadvantage of formal teaching is that the activity is mainly unilateral. In fact, teacher delivers the material and at the same time controls the class and students are responsible for learning. In this method, students rarely can enter into discussion and individual views, enthusiasm, and innovations can not be involved in teaching process. Therefore, the learners gradually will get tired and may loss their desire toward the listening and wish the class be over soon [12-14]. On the other hand, teachers are in a unilateral situation and they may not discover the learning needs of their students, and they can not find out the subjective potential of the students because there is no space for students to interact in teaching-learning process. Formal teaching method in Europe and America is more effective than Iran. In those countries, both teachers and students feel responsible toward teaching and learning respectively. However, In Iran, due to educational anarchies, and social malfunctioning, formal teaching method may not be effective. In Iran, most teachers and university faculties teach for their daily bread, and students try to get a degree. Therefore teachers try to simply and readily deliver some summaries and notes to students and free themselves from educational complexity and cumbersome situations. Students try to memorize some jargon a couple of nights before the exam and then by taking the exam, free themselves from all these trouble and agony. In this situation, the formal teaching method in Iran does not have required efficiency. Therefore, the educational system of Iran is changed to a fallacy system. In general, formal teaching method is boring, monotonous and non persuasive.
3.2. Socratic Method
- The Socratic teaching method [15, 16] is mostly based on major discussion between teacher and student. Teacher raises a question and make student to think about the question. The student tries to give a proper answer to the teacher. Then the teacher with the second question which is some how related to the first question, will drive the student toward the correct answer. This mutual scientific dialogue (question and answer) between teacher and student will continue until the learner or student indirectly and with the guidance of the teacher can find the right answer. This method is interesting, because it develops a potential or incentive in student and learner find himself in the process of learning. In fact this is the learner that should search for the answer (knowledge), mean while he/she can have the advantageous of teacher's knowledge and guidance. In addition, the same method is automatically used among the students of a class, and they test each other with asking scientific questions by which they make each other to think and as a result enhancing their knowledge. Maclean [17], shows that how Socratic approach is used to guide law students. The Socratic approach complements the case method as the classic technique used in legal education to teach students to think like lawyers [18-20]. Krug and Rhaesa [21], argue that the process of Socrates' approach forces student debaters to learn the practices of advocacy, clash, refutation and extension which are critical to advancing in the learning process.Here is an example of Socratic Method: Teacher: Among compounds such as benzene, water and ethylene glycol, which on has higher boiling point?Student after thinking: water! Since it has H-bonding between its molecules!Teacher: Don't you think that benzene and ethylene glycol can also provide H-bonding?Student: Benzene does not have electronegative group, therefore can not make H-bonding. But I do not know if ethylene glycol contains any electronegative group?Teacher: your answer regarding benzene is correct. But ethylene glycol has two hydroxyl groups. Student (after thinking for a while): Excuse me, according to your saying, ethylene glycol has higher boiling point because it has two strong electronegative groups. Is that right? Teacher: Bravo! Now you have found the correct answer.As you see from, dialogue of student and teacher, student all by himself, by trial and error with little help of teacher gradually can find the correct answer. This trend not only increases students' independence and attitude, but also provides a great incentive in students. In this method both teacher and student feel responsible to what they do and the teacher should up to date his knowledge.However, the disadvantageous of this method is that it is time consuming process and can not be used for large group of students. Teacher and students must use a great deal of time to discuss with each other every day. The essence of this paper is that, it offers the practical application of mixed method, i.e., formal plus Socratic method. If we use formal method mentioned above with the Socratic Method in the same framework, we may be able to achieve advantageous of both methods, and the disadvantageous of both will be eliminated too.
3.3. Formal-Socratic Method
- This method may be the best practical one. This method has both, formal and Socratic advantages. Teachers are forced to up to date their knowledge and students would have incentive to search for answers of the questions. It means that responsibilities automatically will be induced in both teacher and student. On the other hand, class will change to an exciting and enthusiastic environment, each student try to overtake other students. Teachers can allocate the first 10 minute of the class for using Socratic Method to answer the question that was raised by them at the end of the previous class. At the end of each class, teacher can raise a new question and ask the students to answer it in the next class. In this way, the students will try by discussion and negotiation with teacher and other students even using books, internet to find the answer of the potential question. This can create a wonderful activity among students for learning. During 25 years of teaching and testing formal-Socratic method of teaching on more than 5000 chemistry students, I found that the student's grades improved more than 15 to 20 percent. Here are some examples for formal-Socratic method:Teacher: our next class will be about "symmetry". What would be the importance and applications of symmetry science?The next class, and the first 15 minutes:First Student: All chemical reactions take place based on molecular or orbital symmetry. It means orbitals with the similar symmetry can overlap.Teacher: bravo, that is right!Second student: All natural organisms some how have symmetry. For example, flowers, animals, plants have symmetry since symmetry helps them to achieve their need better and can perform and act more efficient.Teacher: Bravo! That is right.Third student: All buildings, instruments, cars, for more stability and higher efficiency and strength should have symmetry. Teacher: of course, very good. But why is that?Next student: all planets, stars, earth, they have symmetry.Teacher: right! But why is that?Student: since they are large!Teacher: no, it is not! Some spherical stones in river are not large!Next student: since all planets and stars such as earth and sun are moving around themselves, therefore the best symmetry for this kind of movement is spherical symmetry.Teacher: good, wonderful, it is right. You answered the question very good. Now listen to the general answer and the point that what the symmetry really is: I would say that all transactions, natural events, chemical reactions, physical changes, social, economical and even moral and artistic activities are mostly based on symmetry.Student: It is strange sir! How could moral and artistic aspects be related to symmetry? Teacher: This is a good question, now listen to my answer:"As I said all natural and man made events are based on symmetry. Two individuals can deal with each other if they have mental and thought symmetries. The music is the symmetry of harmonies and rhythm. Poems are symmetric terms and what makes poem beautiful, in fact is its rhyme and symmetry. What makes flowers beautiful, are the symmetry of their petals. What makes human and animals more stable and helps them to perform properly and carry out vital functions are their symmetry. Imagine if one leg of an individual was one meter longer than the other (not symmetrical) then how funny and problematic could that be. Therefore, symmetry is not only a scientific issue but it is also a general and physiological mater. Now let me to start the lesson for today which is about "Symmetry applications in chemistry" and explain to you that how symmetry can play an important role in chemistry and atomic orbitals.Teachers try to push and encourage students into a scientific challenge and endeavor about symmetry a head of starting to give a lecture on the symmetry subject. He uses the Socratic Method as a motive to induce action and incentive, then for teaching speed, he can use formal method for the rest of the time which is remained.In this method, you realize that students move from parts to totality but instead teacher moves from totality to parts. These concepts of “totality” and “parts” are cornerstones in formal-Socratic teaching method.
4. Direction of Learning
4.1. Moving from Parts toward Totality
- There has been a vast research regarding the effect of Gestalt theory on education. Gunay [22] in his paper “gestalt theory and city planning education” claims that, the relationship between the elements of any whole and their restructuring, owe much to the Gestalt theory. He continues that since we are dealing with the whole-part relationship, gestalt teaching surpasses theory of form and become a method of education by itself. Kobery and Bagnall [23], consider the principles of design or composition as harmony, contrast, balance, order, and unity recognizable respectively to similarity, differences, stability, pattern and collectiveness for all parts of the whole. To achieve belonging togetherness of elements three rules are essential: similarity, proximity and continuity [24, 25], and that western education (formal method) has been concerned foremost with words and numbers, complains how such processes detach the chills from sensory experiences.In general, any time the knowledge of an individual about an issue is limited then automatically, his view will turn toward parts side of actuality. In other world, individual can not grasp the essence of the issue. The learner behaves objectively and involves himself more into the parts of the totality and will remain in parts erroneously. After a while, when the knowledge of an individual increases, then he can realize the totality of the issue. Partly attitude is an obstacle for learning process and it takes time until the learner get to know general trend of a system.One of the main reasons for ideas diversity and appearance of different religions, in fact is partly attitude of ancient people. Due to lack of knowledge, each individual would find his God through something. Therefore each one may think right since all of these things are the creation of God. However, a wise man could offer a general and total sight and believe that all the existences are created by God. Therefore, we should make student to think gestalten and moving from totality toward parts. From totality we may reach to the parts prospective easily and precisely very fast but going from parts toward totality is very long and usually accompanied by error. One of the main advantage of formal-Socratic method is that it can strength and develop the totality in learner attitude. All the eminent people and great scientists have gestalten views (believe in totality). Psychologists who believe in gestalten school, they look at total system of existence and they believe that you can not see all aspects, corners and faces of a system or behavior except you look at it in complete and total way. By other words, totality attitude means "insight". A person with totality attitude has insight. Totality has a significant relationship with its ingredients so that you can not find this relationship through knowing only the components. A person with nongestalten views is like a person who put his hands in a dark chamber and he touches some distinct objects and saying that "this one is like trunk of elephant and that is like elephant feet." But he can not realize and has a doubt that there may be an elephant in this chamber. But a person who has gestalten view can see the whole story in its totality. A person who sees the elephant in its totality, then he will see the feet, trunk and relation between them. In most of the time, parts by themselves do not have any significant meaning, but when they come together then they will get a real meaning. For example, m, a, and n alone do not have any meaning. However, when they come together "man" it will provide meaning and the relationship between the components will be clear.We remember from high school, when a teacher for a first time wanted to teach us the equilibrium constant of a reaction. We have many different equilibrium constants such as acidic, basic, formation, solubility and dissociation constants. The teacher used to teach these constants separately in different times and we taught that each of these constants were completely different issues. Since teachers, teaching these constants in parts and separate them, we may think that each one of these constants are different issues. In fact the teacher might have nongestalten insight with respect to the issue and we could not realize the relationship among these constants. In fact, we are learning each constant separately in different period of time, therefore we may think that each constant differs from one another and they do not have any relationship among themselves. The gestalten and nongestalten views should be illustrated by the following examples from chemistry lectures:Example 1: Teacher who has nongestalten views:Teacher: The equilibrium constant of an uncompleted reaction such as Fe(III) with water is:
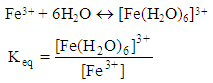 After a couple of months the teacher talks about acidic constants,Teacher: The acidic constant for the following reaction is:
After a couple of months the teacher talks about acidic constants,Teacher: The acidic constant for the following reaction is: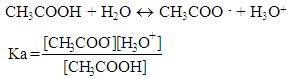 After several months the teacher talk about basic constant,Teacher: The basic constant is:
After several months the teacher talk about basic constant,Teacher: The basic constant is: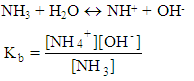 Couple of years later, the teacher talks about formation constants,Teacher: Formation constant for the following reaction is:
Couple of years later, the teacher talks about formation constants,Teacher: Formation constant for the following reaction is: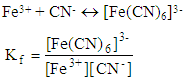 Several months later the teacher talks about solubility constant,Teacher: the solubility constant of an insoluble compound such as AgCl is:
Several months later the teacher talks about solubility constant,Teacher: the solubility constant of an insoluble compound such as AgCl is: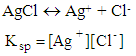 The above subject may be explained much better by a teacher who thinks gestalten. He will simply explain all constants in couple of general sentences such that a learner can grasp the meaning and the relationship between them.Example 2: Teacher who has gestalten views:Teacher: Today, I would like to teach you a new concept and that is the equilibrium constants of reactions. Depend on a reaction and the type of reactants involved in the reaction, we may have different constants. Even though all of them are equilibrium constants, however, because the reactions and mechanism of the reactions are different therefore, different names are used for the constants. For instance, if the primary reactant is a weak acid that react with water, then the equilibrium constant is called acidic constant. By the same way if the primary reactant is a base, then the equilibrium constant is called basic constant. If the primary reactant is a precipitate, and wants to dissociate in water, then the constant is solubility constant. At the end if the reactants react with each other and make a complex, then the equilibrium constant is formation constant. Therefore, for a general reaction such as below:
The above subject may be explained much better by a teacher who thinks gestalten. He will simply explain all constants in couple of general sentences such that a learner can grasp the meaning and the relationship between them.Example 2: Teacher who has gestalten views:Teacher: Today, I would like to teach you a new concept and that is the equilibrium constants of reactions. Depend on a reaction and the type of reactants involved in the reaction, we may have different constants. Even though all of them are equilibrium constants, however, because the reactions and mechanism of the reactions are different therefore, different names are used for the constants. For instance, if the primary reactant is a weak acid that react with water, then the equilibrium constant is called acidic constant. By the same way if the primary reactant is a base, then the equilibrium constant is called basic constant. If the primary reactant is a precipitate, and wants to dissociate in water, then the constant is solubility constant. At the end if the reactants react with each other and make a complex, then the equilibrium constant is formation constant. Therefore, for a general reaction such as below: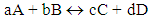 The equilibrium constant is:
The equilibrium constant is: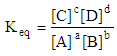 Accordingly we will have the following rules:• if the above reaction is dissociation of a weak acid then Keq = Ka• if the above reaction is a dissociation of a week base then Keq = Kb• if the above reaction is formation of a complex then Keq = Kfor.• and if the above reaction is a solubility reaction of a solid then Keq = KspHere, we see that the teacher’s view of totality, attitudes and sights are vast and looks at the issue with a general prospective. Therefore, by learning the general meaning of equilibrium constant, students can also realize the relationship among these constants and could have a total primacy over the subject. This kind of trend in science would be very crucial for teaching\learning process. Students can grasp and understand parts by realizing first the totality of the fact. However the reverse is not necessarily true. This paper is going to be end by the fact that in real world, teaching\learning process could be a mixture of formal (educationally, highly equipped) and gestalten (consider totality) performance.
Accordingly we will have the following rules:• if the above reaction is dissociation of a weak acid then Keq = Ka• if the above reaction is a dissociation of a week base then Keq = Kb• if the above reaction is formation of a complex then Keq = Kfor.• and if the above reaction is a solubility reaction of a solid then Keq = KspHere, we see that the teacher’s view of totality, attitudes and sights are vast and looks at the issue with a general prospective. Therefore, by learning the general meaning of equilibrium constant, students can also realize the relationship among these constants and could have a total primacy over the subject. This kind of trend in science would be very crucial for teaching\learning process. Students can grasp and understand parts by realizing first the totality of the fact. However the reverse is not necessarily true. This paper is going to be end by the fact that in real world, teaching\learning process could be a mixture of formal (educationally, highly equipped) and gestalten (consider totality) performance. ACKNOWLEDGEMENTS
- I would like to acknowledge the University of Payame Noor of Lamerd, for their financial support and the opportunities that were provided for our research. I sincerely thank Parvaneh Dodman for correction and editing this paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML