-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Education
p-ISSN: 2162-9463 e-ISSN: 2162-8467
2013; 3(2): 113-117
doi:10.5923/j.edu.20130302.01
The Concept of Electronegativity: Approximations and Separations in Chemistry Textbooks
Edson José Wartha1, Camila Maria Andrade dos Santos1, Ricardo Alexandre Galdino da Sil2, e Raildo Mota de Jesus3
1DepartmentofChemistry, Universidade Federal de Sergipe (UFS), Aracaju, Sergipe, Brazil
2Department of Exact Sciences and Earth ,Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil
3DepartmentofExactSciencesand Technologies, Universidade Estadual de Santa Cruz (UESC), Ilhéus, Bahia, Brazil
Correspondence to: Edson José Wartha, DepartmentofChemistry, Universidade Federal de Sergipe (UFS), Aracaju, Sergipe, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this work we seek to identify approximations and separations in relation to the Reference Sciences tothe concept of Electronegativity in Chemistry Textbooks using the Cone Model. We identify that there is a strong standardization of the Electronegativity concept at High School and Higher Education textbooks, as well as ahistorical and decontextualized treatment from the original source. The textbooks analyzed showed a strong standardization of characteristics that, from our point of view, is configured as a horizontal separation in relation to knowledge of reference that can lead students to conceptual errors.
Keywords: Electronegativity, Chemistry Textbooks, Didactic Transposition
Cite this paper: Edson José Wartha, Camila Maria Andrade dos Santos, Ricardo Alexandre Galdino da Sil, e Raildo Mota de Jesus, The Concept of Electronegativity: Approximations and Separations in Chemistry Textbooks, Education, Vol. 3 No. 2, 2013, pp. 113-117. doi: 10.5923/j.edu.20130302.01.
1. Introduction
- According to Lopes[1], the passing of the scientific knowledge to the scholarly context is a process of transformation; after all, if science is a collective production that is socially contextualized, the removal of scientific knowledge from this context implies in its transformation. Further, according to Lopes[2], even before the “arrival” at schools, this transformation begins with epistemological and social purposes.Chevallard[3]states that the teaching of a determined element of knowledge will only be possible if it undergoes some type of “transformation or deformation”. In this sense, he indicates elements that characterize didactic functioning based on the concept of didactic transposition, andthe knowledge taught assumes processes of decontemporization, naturalization, decontextualization (to bring something significant from wisdom knowledge, decontextualizing and then recontextualizingit in a different discourse) and depersonalization. Chevallard[3]statesthat didactic transposition is “the work of fabricating an object of teaching, that is, to make an injection of knowledge produced by wisdom to be an object of scholarly knowledge.”Pinho-Alves[4] points out that the transformations accomplished by means ofdidactic transposition make scientific knowledge accessible, and that these transformations are made by different actors belongingto the diverse social instances related to education – official education agencies, universities, researchers, textbook authors, and teachers, among others. For this author, a transformative process requires the determination or adoption of a starting point or reference point. The reference point or “reference knowledge” adopted is the knowledge produced by scientists according to the statute rules of the community towhich it pertains, submitted to rules and specific language. On the other hand, the knowledge to be taught (scholarly knowledge) also has its own rules during the process of didactic transposition. According to Chevallard[3] these rules will undergo a process of degradation, in which the loss of the original context considering its source occurs through decontextualization and reconstruction. The didactic transposition process, by removing scientific concepts from the historical context of their production and their limiting to restricted definitions, may generate obstacles to the understanding of these same concepts. According to Forquin[5], the scholarly culture (scholarly knowledge) is considered as a “secondary culture” in relation to the “culture of creation or invention.” For this author, scholarly culture is derived and transposed from knowledge, and can be identified through teaching materials, because before arriving at school (high school, higher education) the reference knowledge suffers processes of approximations or separations with the objective of becoming knowledge to be taught. Therefore, such approximations and separations are part of the didactic transposition process, which do not mean that there are easy tasks to be accomplished, because the act of transforming scientific knowledge into didactic content - conserving its complex theories and without losing its properties and characteristics - may be considered the largestchallenge for textbook authors, teachers and the school. Thus, it is necessary to consider not only the characteristics of the knowledge themselves, but also the characteristics of the student, and his or her capacity for reasoning and previous knowledge. In order to be able to differentiate the types of separations found, we use a tool developed by Franzolin[6], by means of which the separations are classified into two categories, both derived from didactic transposition: vertical distancing, which originates from the transposition of the scientific knowledge to each level of teaching, being necessary to facilitate the learning for students of different age groups. This is represented by a central axis, and all knowledge that is inserted within the cone that surrounds it would originate from a separation from this category (Figure 1). The knowledge that has a greater rigorousness in relation to the referenceis inserted on the axis represented in Figure 1 by the straight V line. The knowledge that is located within this cone (exemplified by the dotsin Figure 1) is also found there due to its rigorousness, or accuracy, in relation to the reference; however, its rigor varies according to the academic age component; the other type of separation would be the horizontal one, referring to the separation in relation to the determined axis by the rigorousness, and, therefore, generates knowledge that is found outside the cone that surrounds it. This skill is used by those who teach to facilitate the learning, but it is not related to the academic age group. This separation would arise from the flexibility of the knowledge taught in relation to the rigorousness related to the reference science. However, it is important to clarify that the knowledge originating from this separation is not reduced to conceptual errors, even though it can also be in this category, but it is knowledge that can have different natures.
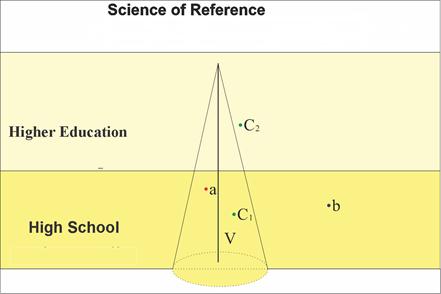 | Figure 1. Representation of the possible types of separation found among knowledge taught at the different school levels and those presented by the reference (adapted by [6]) |
2. Methodology
- In order to perform the comparison between knowledge of electronegativity found at High School and Higher Education textbooks in relation to the reference knowledge and its approximations and separations, a table was created with the frequencies of the separations found in the diverse contents or areas, which were then confronted with the reference knowledge. In this research, reference knowledge is considered as the knowledge of the scientists and which is found in periodicals. The textbooks used in the analysis are represented in Table 1.
3. Results
- In this analysis we analyzethe concept of electronegativity proposed by J.J. Berzelius in 1811, when developing the electrochemical theory of bonding (also known as dualistic theory). Berzelius organized the simple bodies in decreasing order of electronegativity, this series being defined due to the manner in which the elements bond in the compounds which defined it as being the capacity that an atom has to attract electrons to it[8]. In 1931, Linus Pauling reformulated and amplified the concept of electronegativity, proposing a scale of electronegativity. Pauling sought to determine differences between the energies of homonuclear bonds and heteronuclear bonds (between different atoms), assuming that if two homonuclear diatomic molecules interact to form diatomic heteronuclear molecules, the bond energy of the latter would be an average of two homonuclear bond energies in the original molecule (Postulating from the addition of normal, covalent bonds). However, Pauling observed that the real heteronuclear bond energy was greater than the average expected, and that this energy increased as the atoms became different in relation to a property, which chemists called electronegativity. Such a property was defined as the power of an atom in a molecule to attract electrons to it, and as the bond energies refer to molecules in a gaseous state, electronegativity also refers to isolated molecules [9].As a consequence of the work realized by Pauling, only differences of electronegativity were defined. According to Santos, Silva and,Wartha [10], after the work of Pauling, severalstudies on electronegativity were carried out, and other scales of electronegativity were proposed based on different atomic parameters and physical properties. Among these are the scales of Müllikenin 1934, Allred-Rochow in 1958 and Sanderson in 1951[10].Mülliken proposed a scale of absolute electronegativity, in which he suggested that this absolute electronegativity would be the mathematical average of the energy necessary to remove an electron from an atom in the gaseous phase (ionization energy) and energy releasedwhen an electron is added to an atom in the gaseous phase (electron affinity). Mülliken suggested that these values should correspond to a state of appropriate valence; that is, the electronegativity would depend on the oxidation state of the element [11]. Allred and Rochow considered that electronegativity was related to the electrostatic force experienced by an electron on the valence layer of the atom caused by its effective nuclear charge. They considered electronegativity as a measurement of the effectiveness of the nuclear charges on the outermost empty orbitals and that, therefore, an intimate relationship would exist between atomic structure and this property. Thus, this property cannot be interpreted solely as a number, but also as a consequence of the atomic structure. The fact that it becomes more distinct when the blinding effect of the nuclear charge from an atom is analyzed and it is perceived that the outermost electrons are not as effective as the innermost, thereby allowingthe effective nuclear charge to interfere in the number of valence electrons from a given atom[12]. Sanderson pointed outthe relation between electronegativity and atomic size. He considered electronegativity as a function of the relative density of the electron cloud around the nucleus of the atom, recognizing that the natural tendency of some atoms with high electronegativity is to acquire partial negative charge, causing expansion of the electrosphere to a less compact condition (less electronic density). The natural result of the partial loss of electrons by an atom of low electronegativity is the contraction of the electrosphere to a more compact condition (greater electronic density)[13]. Pearson suggested that the different scales of electronegativity have distinct applications and that each one is correct within their own areas of application. A scale of electronegativity should adequately reflect the particularity of the elements, and explain in a satisfactory manner various physical and chemical properties of the compounds. Pauling’ scale is the most extensively used, because it has been effectively used to predict the polarity of a bond, solubility and the fusion point of compounds. The reliability of the other scales is generally verified by means of the comparison with the original scale. Thus, when working with atomic electronegativity, the values proposed by Pauling in 1932 and revised by Allred and Rochov in 1958 are recommended [13].The concepts of electronegativity found in the textbooks are listed in Table 2.
|
4. Conclusions
- We identified that the majority textbooks, both for High Schooland Higher Education, show the concept of electronegativity in an ahistorical and decontextualized manner. The knowledge taught appears as knowledge without producers, without origin, without place, transcendent of time, teaching only the result, isolating them from the history of construction of the concept, removing them from the group of problems and questions from which they originated. In this perspective of teaching, the concept of electronegativity becomes inadequate to the reality into which it is inserted, because the information present in the textbooks, generally speaking, does not seek to explain the relation between electronegativity and other periodical properties. We also verify that the textbooks useonlyLinus Pauling’s definition of electronegativity of, not taking into account the contributions made by Mülliken, Allred-Rochow and Sanderson.One can conclude that both High School Textbooks andHigher Education Textbooks show a strong standardization of characteristics that, from our point of view, is a horizontal separation in relation to knowledge of reference that can lead students to conceptual errors.Thus, it is proposed that the treatment of the concept of electronegativity be performed within a historical context establishing connections between electronegativity and the concepts with which this is constituted in an inter-relational base.
References
| [1] | LOPES, A. R. C. Conhecimento escolar: ciência e cotidiano. : edUERJ, 1999. Rio de Janeiro.Eduerj, 1999. |
| [2] | LOPES, A. C. Currículo e Epistemologia. Ijuí: Unijuí, 2007. Ijuí - RS - Brasil : Ed. Unijuí, 2007. |
| [3] | CHEVALLARD, Y. La transposition didactique: du savoir savant au savoir enseigné .Paris :Seilc, 1991. |
| [4] | PINHO ALVES, J. Caderno Catarinense de Ensino de Física. 2001, Vol. 2, n.17, 174-188. |
| [5] | FORQUIN, J. C. Teoria e Educação. 1992, Vol. 5, 28-49. |
| [6] | FRANZOLIN, F. Conceitos de Biologia na educação básica e na acadêmica: aproximações e distanciamentos. São Paulo: Dissertação de mestrado - FEUSP 2007. |
| [7] | BRASIL. Ministério da Educação. Guia de livros didáticos: PNLD 2012: Química. Brasília: 2011. |
| [8] | RHEINBOLDT, H. História da balança. A vida de J.J. Berzelius. São Paulo : Edusp, 1988. |
| [9] | PAULING, L. The nature of the Chemical Bond. 3. Cornell : Ed. Ithaca: Cornell University Press, 1960. |
| [10] | SANTOS, C. M. A. D., SILVA, R. A. G. D. e WARTHA, E. J. Quim. Nova. 2011, Vol. 34, n.10, 1846-1851. |
| [11] | MÜLLIKEN, R. S. J. J. Chem. Phys., 1934. 782. |
| [12] | ALLRED, A. L.; ROCHOW, E. G. J. Inorg. Nucl. Chem, 5, 1958. 264. |
| [13] | SANDERSON, R. T. A. Science, 114, 1951. 670-682. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML