-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2020; 9(2): 43-47
doi:10.5923/j.diabetes.20200902.03
Received: July 8, 2020; Accepted: July 24, 2020; Published: August 15, 2020

Zonulin as a Responsive Marker for Lifestyle Intervention: A Preliminary Study
Satria Abi Dileyon 1, Marselina Irasonia Tan 1, Felicia Kartawidjajaputra 2
1School of Life Science and Technology, Bandung Institute of Technology, Bandung, Indonesia
2Health & Nutrition Science Department, Nutrifood Research Center, Jakarta, Indonesia
Correspondence to: Felicia Kartawidjajaputra , Health & Nutrition Science Department, Nutrifood Research Center, Jakarta, Indonesia.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Insulin Resistance (IR) is the onset pathological condition that emerges before the occurrence of type 2 diabetes mellitus (T2DM). The development of IR condition is very difficult to observe. The initial stage of this condition does not show significant symptoms. Therefore, biomarkers are needed to detect this condition early and accurately. Zonulin is a protein that plays a role in regulating intestinal permeability and might be potential as an IR biomarker. Recent studies have shown the association of zonulin with T2DM and other metabolic diseases. However, there have been no studies showing zonulin profile in subjects at risk of IR and its response towards healthy lifestyle intervention. Therefore, this study aimed to determine the profile of zonulin in the blood plasma of subjects at risk of IR (n = 16 persons) relative to T2DM subjects (n = 3 persons) and healthy subjects (n = 42 persons), as well as changes after the intervention of red rice consumption and physical exercise for 3 months. The intervention included red rice consumption five days per week (250 grams / serving), physical exercise 150 minutes per week, and counseling once every two weeks. Plasma zonulin was measured semi-quantitatively using the sandwich ELISA method. The results showed a significant difference in plasma zonulin OD (p <0.001) between the three groups. In T2DM and IR subjects, plasma zonulin profile was 9.9 times (2.437 ± 0.290) and 3.1 times (0.767 ± 0.575) higher than healthy subjects (0.246 ± 0.167) respectively. After 3 months of red rice consumption and physical exercise, there was no significant change in glycemic profile, in both IR and healthy subject. Interestingly, the plasma zonulin in IR subjects decreased significantly (p = 0.019), to an average value (0.276 ± 0.106) which was almost the same as the healthy subjects (0.264 ± 0.170). Thus, zonulin appeared to be more responsive as a marker of lifestyle intervention than the commonly used glycemic parameters.
Keywords: Zonulin, Insulin Resistance, Diabetes Mellitus, Lifestyle Intervention, Early Marker
Cite this paper: Satria Abi Dileyon , Marselina Irasonia Tan , Felicia Kartawidjajaputra , Zonulin as a Responsive Marker for Lifestyle Intervention: A Preliminary Study, International Journal of Diabetes Research, Vol. 9 No. 2, 2020, pp. 43-47. doi: 10.5923/j.diabetes.20200902.03.
Article Outline
1. Introduction
- Diabetes mellitus is the 9th biggest cause of death in the world. The number of people with diabetes mellitus has increased fourfold over the past 30 years. One in eleven adults are predicted to have diabetes mellitus. The epidemic of diabetes mellitus is observed to be the highest in Asia [1]. Indonesia has the 7th highest number of people with diabetes mellitus in the world. People with diabetes mellitus in Indonesia are estimated at 10 million in 2015 [2]. Of the total population of people suffering diabetes mellitus, an estimated 87-91% have type 2 diabetes mellitus (T2DM) [3]. Insulin resistance (IR) is the initial pathological condition that precedes type 2 diabetes mellitus (T2DM). The development of IR condition is very difficult to observe because it does not show significant symptoms [4]. Symptoms usually indicate that the patient has entered the chronic stage of T2DM and becomes more difficult to treat. Therefore, a biomarker is needed to detect this disease early and accurately.Zonulin is a regulatory protein that plays a role in increasing gut intestinal permeability. The presence of zonulin is able to regulate tight junctions, create oxidative stress, and trigger an inflammatory response [5]. Several studies have shown the potential of zonulin as a biomarker for health status related to various metabolic diseases. High zonulin profile in the blood was observed in obese [6], T2DM [7], and non-alcoholic fatty liver disease subjects [8] compared to the healthy group. Thus, zonulin might be potential as a biomarker of IR condition.Unhealthy eating habits, such as high sugar and fat consumption, and sedentary lifestyles are the risk factors for developing T2DM. Although genetic factors also play a role in increasing the risk of T2DM, unhealthy lifestyles are important factors that determine the etiology of this disease. The risk of T2DM could be reduced through a healthy diet, maintaining ideal body weight, regular daily exercise, and avoiding smoking and alcohol [9,10]. However there have been no studies showing zonulin profiles in subjects at risk of IR; and their response to healthy lifestyle intervention.In this study, an analysis of plasma zonulin profile in subjects at risk of IR was conducted, relative to T2DM and healthy subjects. Lifestyle intervention such as consumption of red rice and exercise was also conducted to determine the changes in the plasma zonulin profile in IR and healthy subjects. This study was expected to provide an initial description of the plasma zonulin profile in subjects at risk of IR, and to assess the response of zonulin as biomarker for lifestyle changes to prevent further pathogenesis of IR condition.
2. Materials and Methods
- Study participants were Indonesian urban citizens selected from employees of PT Nutrifood Indonesia. A total of 61 participants consisting of 27 men and 34 women were involved in this study. This research design consisted of cross-sectional and intervention study. Intervention stage was carried out at Nutrifood Research Center, PT Nutrifood Indonesia, Jl. Rawabali II No. 3 Jakarta which lasted from September to December 2017. Meanwhile, measurement of plasma zonulin was carried out at the Laboratory of Animal Structure and Development at School of Life Science and Technology - Bandung Institute of Technology, from January to June 2018. This study was approved by the Atma Jaya Catholic University's Research Ethics Commission with ethics number FR-UAJ-26-13 / R0.
2.1. Research Design
2.1.1. Cross-sectional Study
- This study was conducted to compare the plasma zonulin profile in subjects at risk of IR with HOMA-IR greater than 1.55 [11] (n = 16 persons, 11 males), T2DM subjects with fasting plasma glucose greater than 125 mg / dL [12] (n = 3 persons, all males), and healthy subjects with HOMA-IR values less than or equal to 1.55 (n = 42 persons, 13 males). The inclusion criteria were adults aged 25-50 years and no smoking habits. The exclusion criteria were pregnant and breastfeeding women, and the consumption of prescription medicine.
2.1.2. Intervention Study
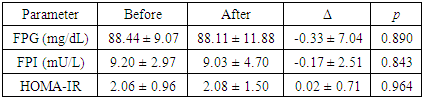
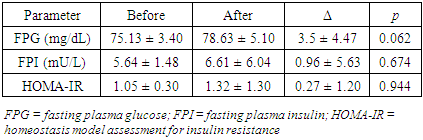
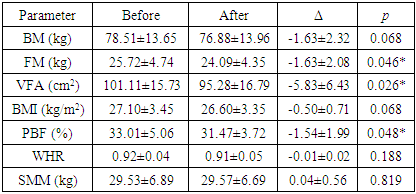
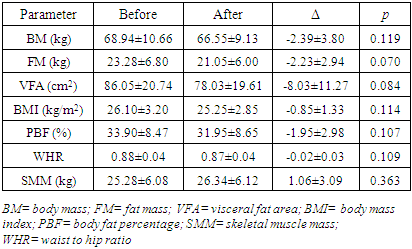
- This study was conducted for 12-weeks using subjects at risk of IR (n = 9 persons, 6 males) and healthy subjects (n = 8 persons, 3 males). At week 0, participants were measured based on the anthropometric parameters, blood biochemical parameters, total calorie, and nutritional intake (through 3 x 24 hour food recall). Anthropometric parameters include the body fat and skeletal muscle mass were measured using the InBody 720 device. The measurement of the blood biochemical parameters include fasting plasma glucose and fasting plasma insulin were performed by commercial laboratory. Participants were also asked to complete the International Physical Activity Questionnaire (IPAQ). The intervention included recommending red rice consumption five days per week (250 grams / serving) [13], sports activity 150 minutes per week [14], and counseling once every two weeks [15]. Nutritional intake and IPAQ were re-measured in the 6th and 12th week to assure all participants followed the intervention guideline. Blood biochemical and anthropometry parameters were re-measured in the 12th week.
2.2. Plasma Zonulin Measurement
- The plasma zonulin concentration was measured using the human zonulin ELISA kit (MyBiosource), and was performed on plasma samples obtained at week 0 and week 12. The measurement was repeated twice.Zonulin specific antibodies had been coated on microplates. Blood plasma samples were added as much as 100 μl into each well, then closed using adhesive strips. The microplates were then incubated at 37°C for 2 hours. The liquid from each well was discarded. Biotin-conjugated antibodies (1x) were added as much as 100μl into each well and incubated for 1 hour at 37°C. Then the liquid in the well was removed and rinsed with a wash buffer (200 μl) and allowed to stand for 2 minutes then discarded. This rinsing procedure was repeated 2 times. One hundred µl of horseradish peroxidase (HRP) that was conjugated with avidin (1x) was added to each well and covered with adhesive strip. Then, the samples were incubated at 37°C for 1 hour. The rinsing procedure was carried again for 5 more times. The 90 μl tetramethylbenzidine (TMB) substrate was added to each well and incubated for 15 minutes at 37°C (avoided from light). A total of 50 µl stop solution was added to each well. The sample was read using a microplate reader at a wavelength of 450 nm within 5 minutes.
2.3. Statistic Analysis
- The results were analyzed using the SPSS Statistics version 25 application (IBM, Armonk, New York, United States). In the cross-sectional study, data analysis was conducted using the Kruskal-Wallis test and Mann Whitney U test, while the results of the intervention study were analyzed using paired T-test. Secondary data which includes anthropometric parameters and blood biochemistry parameters was processed using a paired T-test or Wilcoxon signed-rank test to determine differences in profiles before and after intervention.
3. Results and Discussion
3.1. Plasma Zonulin Profile in Subjects at Risk of Insulin Resistance, Type 2 Diabetes Mellitus, and Healthy Subjects
- The presence of zonulin in blood plasma was analyzed semi-quantitatively using the sandwich ELISA method. The ELISA method provides an output in the form of optical density (OD) values. Analysis of plasma zonulin profiles was conducted in 16 persons at risk of IR, 3 persons of type 2 diabetes mellitus (T2DM), and 42 healthy persons. The optical density of plasma zonulin profile of the three groups is shown in Figure 1.
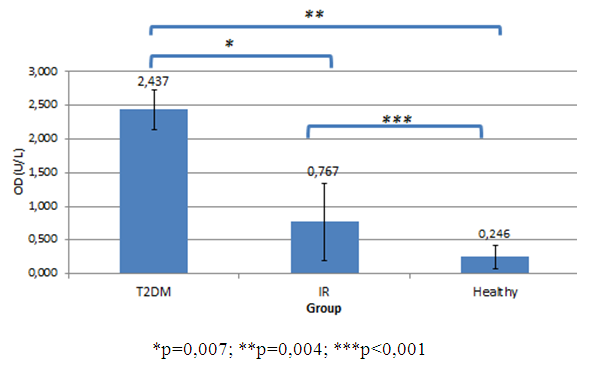 | Figure 1. Plasma zonulin profiles of T2DM, IR, and healthy subjects |
3.2. Blood Biochemical Profile, Anthropometric Parameters, and Plasma Zonulin Profile after the Intervention of Red Rice Consumption and Exercise
- IR condition could be prevented by a healthy lifestyle before becoming a severe condition of diabetes mellitus. Healthy food consumption accompanied by exercise could be a solution for IR condition. Meanwhile, diabetes mellitus need further medical treatment. We would like to see the changes of blood biochemical profile, anthropometric parameters, and plasma zonulin profile after lifestyle intervention in IR and healthy subjects. Changes in blood biochemical profiles after the intervention of red rice consumption and physical exercise can be seen in Table 1 (for IR subjects) and Table 2 (for healthy subjects).
|
|
|
|
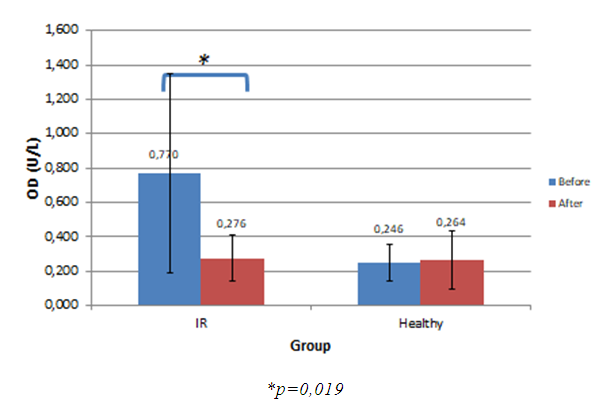 | Figure 2. Plasma Zonulin Profile Before and After Intervention |
4. Conclusions
- This study indicated that the profile of plasma zonulin in subjects at risk of IR was observed to be 3,2 times lower than in T2DM subjects and 3,1 times higher than in healthy subjects. To our knowledge, this was the first study to investigate the zonulin profile in IR subjects, since the previous studies focused in healthy, T2DM, or other digestive disorder. Furthermore, zonulin appeared to be more responsive towards lifestyle intervention compared to commonly used glycemic parameters. Thus, zonulin has the potential to be explored as an early marker in area of diabetics study.
ACKNOWLEDGEMENTS
- Thank you to PT Nutrifood Indonesia for providing and funding this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML