-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2020; 9(2): 23-28
doi:10.5923/j.diabetes.20200902.01

Treatment Initiation with Insulin Glargine 100 U/mL in Type 2 Diabetes Patients Uncontrolled on Oral Anti-diabetic Agents in Jordan: Results of the NewLan Real-Life Study
Rashad Nasser1, Mwafaq Alhyari2, Jihad Haddad3, Sami Haddad4, Chahine Fadel5, Nisrine Sabra6, Jean Tannous7
1Internal Medicine Department, Endocrinology Division, the Italian Hospital, Italian Hospital St, Amman, Jordan
2Prince Hamza Hospital, Fakhr Ad Din Ar Razi 12, Amman, Jordan
3Bader Medical Center, Zahleh St., Amman, Jordan
4Jabal Amman, Fourth Circle, Amman, Jordan
5Medical Director Levant, Sanofi, Beirut, Lebanon
6Clinical Operations Manager, Sanofi, Beirut, Lebanon
7Medical Manager, Sanofi, Beirut, Lebanon
Correspondence to: Rashad Nasser, Internal Medicine Department, Endocrinology Division, the Italian Hospital, Italian Hospital St, Amman, Jordan.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background and Aims Insulin glargine is one of the most commonly used basal insulin analog with a well-established safety and efficacy profile. In the present NewLan study, we aimed to assess the safety and efficacy of insulin glargine 100 units/mL (Gla-100) in patients with poorly controlled type 2 diabetes mellitus (T2DM) despite being on oral anti-diabetic (OAD) medications. Methods NewLan was a national, multicenter, open-label, uncontrolled, prospective six months phase IV clinical trial. Patients with T2DM who were uncontrolled (HbA1c >7.0%) on OAD therapy and commencing Gla-100 therapy were enrolled in 13 sites in Jordan. The primary outcome was the change of HbA1c from baseline to 6-month. Results Overall, 240 patients were included and started treatment with Gla-100. Mean HbA1c was decreased significantly (p <0.001) by -2.09 ± 1.54%, after 6 months. Similarly, mean baseline FBG decreased significantly (p <0.001) from 224.9 ±5.7 to 128.5 ±3.9 mg/dL after 6 months). The percentage of patients achieving target HbA1c (<7.0%) at 6 months was 25.4% (n=45). 90 patients (37%) experienced at least one hypoglycemic event. Only 6 of these symptomatic events were severe (2.5%); none of the hypoglycemic events was classified as serious. Conclusion In a practical care setting in Jordan, initiation of Gla-100 as add-on therapy to OADs effectively improved glycemic control in T2DM patients after six months and was well tolerated with a low incidence of adverse events including hypoglycemia. This simple basal insulin regimen may facilitate effective insulin use in routine medical practice, improving achievement of recommended standards of diabetes care.
Keywords: Insulin Glargine, Type 2 Diabetes, Jordan
Cite this paper: Rashad Nasser, Mwafaq Alhyari, Jihad Haddad, Sami Haddad, Chahine Fadel, Nisrine Sabra, Jean Tannous, Treatment Initiation with Insulin Glargine 100 U/mL in Type 2 Diabetes Patients Uncontrolled on Oral Anti-diabetic Agents in Jordan: Results of the NewLan Real-Life Study, International Journal of Diabetes Research, Vol. 9 No. 2, 2020, pp. 23-28. doi: 10.5923/j.diabetes.20200902.01.
Article Outline
1. Introduction
- Diabetes mellitus (DM) is a major public health burden that is ranked as the ninth major cause of death worldwide; the World Health Organization (WHO) has declared diabetes the epidemic of the 21st century [1]. The incidence of diabetes mellitus, particularly type 2 diabetes is increasing dramatically across the world because of increasing obesity, sedentary lifestyle and population aging, and is the cause of substantial morbidity and mortality [2]. More than 400 million people are currently living with diabetes worldwide, and that figure is expected to rise to more than 600 million by 2045. This means there will be 50 percent more people living with diabetes in the next 25 years. This rise is predicted to occur virtually in every nation, with the greatest increases expected in developing countries. Furthermore, the International Diabetes Federation (IDF) estimates that at least 50% of people with diabetes are unaware of their condition. [2,3]. Type 2 diabetes mellitus (T2DM) is the most common type of diabetes that is characterized by the development of insulin resistance as well as the progressive failure of pancreatic beta-cell function, with subsequent hyperglycemia [4]. In addition to the primary pathology, common comorbidities such as renal, cardiovascular disease, and stroke are usually associated with T2DM [5]. The United Kingdom Prospective Diabetes Study (UKPDS) in subjects with type 2 diabetes support the position that early treatment of diabetes with tight blood glucose control can decrease the morbidity and mortality of the disease by decreasing its chronic complications. Therefore the major goal of treatment of diabetic patients is to achieve good (near normal) metabolic control, thus preventing the onset of the long-term complications [6]. Oral anti-diabetic agents (OADs) are generally effective drugs for achieving glycemic control and preventing both microvascular and macrovascular complications of T2DM [7]. However, recent global figures showed that the majority of patients with a longer duration of diabetes remain poorly controlled with oral agents [8].Insulin therapy is a well-established strategy to reduce both micro and macrovascular complications in patients with poorly controlled T2DM [9]. However, use of insulin, which could improve glycemic control, is often long delayed and not aggressive enough. The reluctance to initiate insulin therapy seems partly due to its perceived complexity and fear of hypoglycemia, which may be the greatest barrier [10]. Insulin glargine 100 units/mL (Lantus®) is a long-acting basal human insulin analogue which reportedly shows no remarkable plasma insulin peaks, and physiologically mimics the normal basal insulin concentrations [11]. In patients with poorly controlled T2DM, insulin glargine 100 units/mL was previously reported to improve glycemic control and achieve a target HbA1c level of less than 7.0% [12], in addition to reducing the risk of hypoglycemia, especially nocturnal hypoglycemia [13]. Compared to NPH insulin, insulin glargine 100 units/mL was superior in controlling glycemic status and reducing the risk of hypoglycemia among insulin-naïve patients with T2DM [14-16].The prevalence of diabetes in Jordan was reported to be 17.1% in 2008, with a progressive increase in its risk [17]. According to the International Diabetes Mellitus Practice Study (IDMPS) wave 5 in Jordan [18], around half of T2DM patients (54.4%), who were treated with insulin, received the premix insulin with only 10% of patients on premix insulin achieving the targeted glycemic control HbA1c < 7%. Therefore, the present real-life study aimed to provide reliable data from Jordan regarding the safety and efficacy of initiation and titration of basal insulin Gla-100 in T2DM patients poorly controlled on OADs.
2. Materials and Methods
- We followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology Statement) guidelines during the preparation of this prospective cohort study [19]. The study was conducted in full accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki, and data for each patient were collected only after obtaining signed written data release forms.
2.1. Study Design, Setting, and Follow-up
- This study was a national, multicenter, open-label, uncontrolled, prospective six months phase IV clinical trial performed between November 2015 and May 2017. The study was conducted in 13 diabetic centers across Jordan to study the effect of Gla-100 on T2DM patients. A total of 242 patients were enrolled for a period of six months and a total of three visits (visit one at baseline, visit two after three months of treatment and visit three after six months of treatment).
2.2. Eligibility Criteria of the Study Participants
- We enrolled both male and female adults patients if they met the following criteria: 1) patients with established T2DM which was uncontrolled with previous therapy as evident by the last glycosylated hemoglobin (HbA1c) value of >7% within the last one month before study entry; 2) Insulin naïve patients who remained uncontrolled after one or a maximum of two lines of therapy including monotherapy (Metformin alone or any OADs) and/or dual therapy (any OADs combination), at maximum tolerated dose in the last 3 months; and 3) patients who agreed to sign the informed consent. We excluded patients with history of impaired renal function defined as serum creatinine >135 μmol/l (>1.525 mg/dl) in men and >110 μmol/l in women (>1.243 mg/dl), pregnant or lactating women (women of childbearing potential must have a negative pregnancy test at study entry and a medically approved contraception method at physician’s discretion), treatment with systemic corticosteroid within 3 months prior to study entry, and patient participation in other clinical trials at study entry.
2.3. Study Treatment
- Insulin Gla-100 (Lantus®), is a clear and colorless solution for injection in a pre-filled SoloSTAR® pen (3 mL), subcutaneous use, administered for 6 months once a day, in the evening, at dinner, or at bedtime. Insulin Gla-100 was added to the patient’s previous anti-diabetic treatment: metformin and any other OADs (at the same dosage as prior to study entry). The starting dose of insulin Gla-100, as per the ADA/EASD Consensus Algorithm, was 0.1 - 0.2 unit/kg of body weight, although larger amounts (0.3–0.4 U kg–1 day–1) were administrated in the more severely hyperglycemic patients. The insulin Gla-100 was injected subcutaneously, once daily, in the evening at dinner or bedtime (at the same time each evening). The time of injection remained the same during the whole study. The patients were provided with identical self-monitoring of blood glucose (SMBG) machines and educated at the sites of the study on how to monitor their blood glucose, titrate their own insulin dose, and record their diaries. According to the ADA/EASD Consensus Algorithm, most patients can be taught to up-titrate their own insulin dose based on several algorithms: 1-2 units (or increments of 5-10% in higher doses) to the daily dose once or twice weekly if the fasting levels are above the pre-agreed target.
2.4. Variables and Data Collection Methods
- At the initial assessment visit, data on patient demographics, clinical characteristics, and prior treatment were collected. In addition, the values of fasting blood glucose (FBG), HbA1c, and insulin daily dose were collected at baseline, three months, and six months later. Safety outcomes were measured throughout the course of treatment. All adverse events, whether related to insulin Gla-100 or not, were recorded from the first day of insulin Gla-100 administration. The seriousness of the adverse events and corrective medications needed were also monitored.
2.5. Study Outcomes
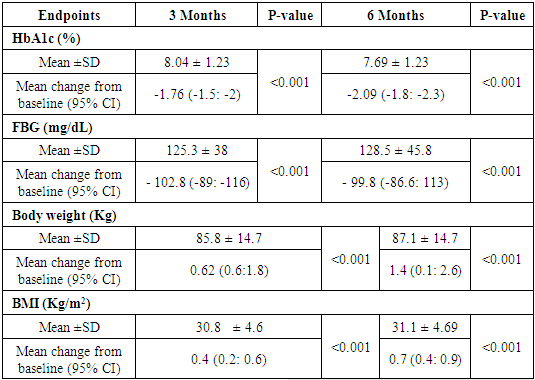
- The primary outcome was the change of HbA1c from baseline to the 6-month follow-up visit. While the secondary outcomes were the percentage of patients achieving the target of HbA1c <7.0% at 6 months, the change in FBG values from baseline and the relative change from baseline to the 6-month follow-up visit, the change in dose of insulin glargine 100 units/mL at 3 months and 6 months, and safety outcomes.
2.6. Statistical Methods
- All variables recorded during the study were summarized. Absolute and relative frequencies were provided for categorical variables. Mean and standard deviation were provided for continuous variables. For quantitative variables, a paired t-test was used to in case of normal distribution, while a Wilcoxon signed-rank test otherwise. A p-value of less than 5% was considered statistically significant. All statistical tests were performed using SPSS program version 25.
3. Results
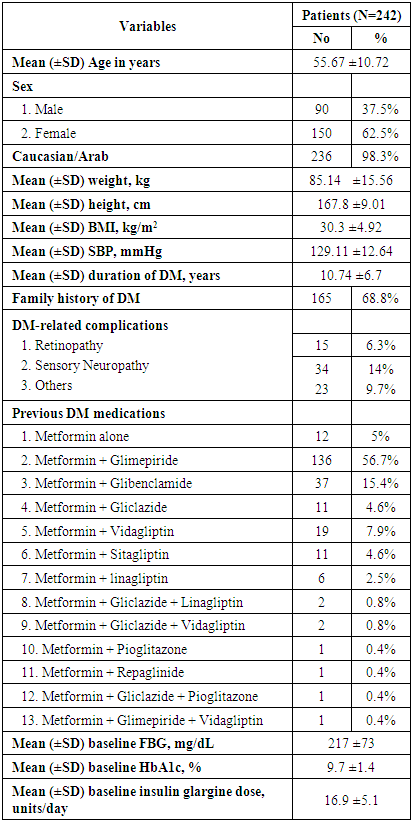
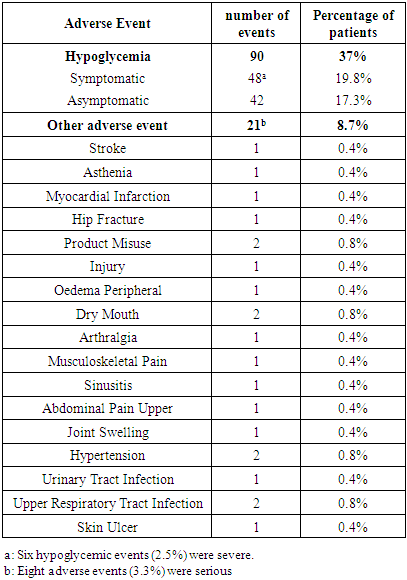
- In the present multicenter prospective study, 242 patients were screened for eligibility, from which 240 patients were eligible and started study’s treatment. The mean age of the included patients was 55.67 ±10.72 years. In addition, the majority of patients were males (62.5%) and Caucasian/Arab (98.3%). The average weight of the included patients was 85.14 ±15.56 kg; while the mean BMI was 30.3 (range 19.2 -44.9) kg/m2. In terms of history of DM, 165 (68.8%) patients had a positive family history of DM, while the mean duration since the diagnosis of DM was 10.74 ±6.7 years. Seventy-two (30%) patients had DM-related complications in which sensory neuropathy (14.2%) was the most common complication. Additionally, 93 (38.8%) patients had medical or surgical comorbidities which were hypertension (42%), dyslipidaemia (32%) and cardiovascular disease (10%). In terms of previous antihyperglycemic therapy, 56.7% of the patients were on metformin plus glimepiride combination and only 5% received metformin alone; the average metformin dose was 2133 ±525mg. With regard to insulin Gla-100, the mean starting dose was 16.9 ±5.1 units/day (range 7-30 units/day). at baseline. In addition, the mean HbA1c was 9.7 ±1.4 % and the mean FBG was 217 ±73 mg/dL at baseline. (Table 1).
|
|
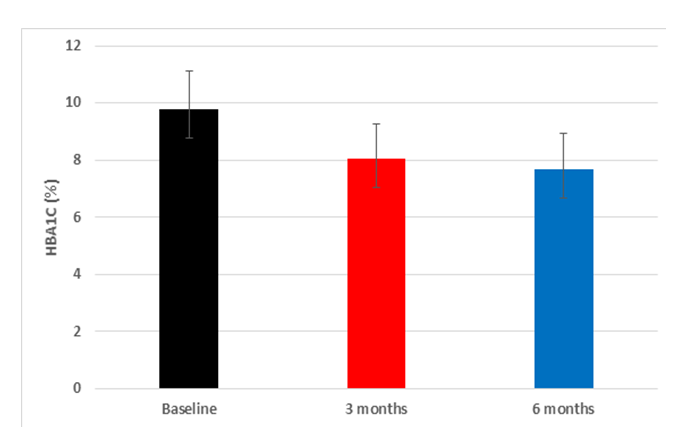 | Figure 1. The mean and standard deviation of the HbA1c over the study periods |
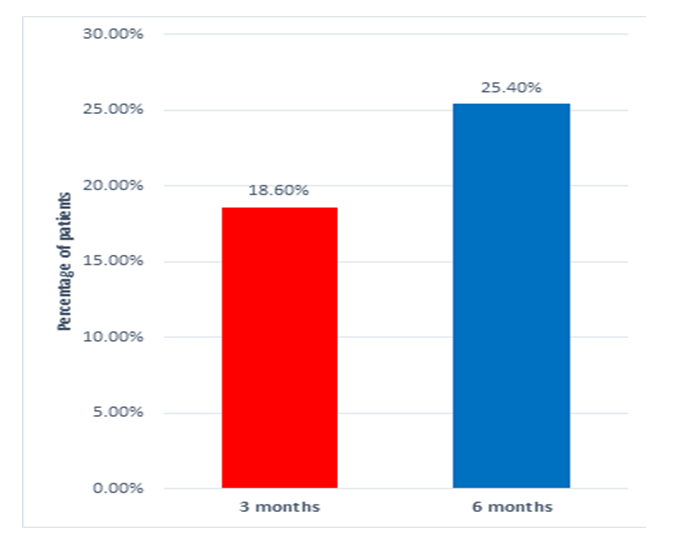 | Figure 2. Percentage of patients who achieved the target HbA1C <7.0% |
|
4. Discussion
- In patients with poorly controlled T2DM, insulin Gla-100 was previously reported to improve glycemic control and reduce the risk of hypoglycemic events. In addition, there is a growing body of evidence that supports the clinical and economic benefits of insulin Gla-100 in real-world settings [20,21] but the published literature lacks real-life data about the safety and effectiveness of insulin Gla-100 in the management of poorly controlled T2DM patients in Jordan.In the present prospective real-life study, insulin Gla-100 was effective for improvement of glycemic status in Jordanian patients with poorly controlled T2DM, both HbA1c and FBG levels decreased significantly at the end of follow-up. Insulin Gla-100 led to a significant improvement in glycemic status at the end of treatment, the average HbA1c level decreased significantly by -1.7591 ±1.5% and -2.09 ±1.54% at the end of third and sixth month of treatment, respectively (p <0.001); moreover, 25.4% of the patients achieved the targeted HbA1c (< 7%) at the end of the study period. The FBG levels showed a significant reduction as well. The insulin Gla-100 showed a well-tolerable safety profile with less than 1% of the included patients experienced severe symptomatic hypoglycemia.In concordance with our findings, a recent prospective observational study from Egypt (Khatab and colleagues) [22] showed poorly controlled T2DM patients who received insulin Gla-100 with or without short-acting insulin had a significantly decreased HbA1c levels from 9.6±1.3% at the baseline to 7.3±0.9% at 6 months and around 35% of the patients achieved the targeted HbA1c <7%. Other regional real-life study by Chraibi and colleagues [23] reported a significant reduction in mean HbA1c level following six months of treatment with insulin Gla-100 in Moroccan patients with poorly controlled T2DM; while 32% of the patients achieved the targeted HbA1c level. Moreover, Florenţiu and colleagues reported that nearly 33% of T2DM reached the targeted HbA1c after 3-6 months of treatment with insulin Gla-100 [21].The published literature shows the long-term beneficial effect of insulin Gla-100 as well; Charbonnel and colleagues [24] reported that insulin Gla-100 significantly increased the proportions of patients with HbA1c <7% to 39% in insulin naïve patients and 34% in insulin-treated patients, following 12 months of treatment. Another study reported that insulin Gla-100 plus OADs significantly reduced HbA1c after 9 months of treatment [25].Additionally, the current published literature suggested that insulin glargine 100U/mL is more effective than regular insulin in controlling the glycaemic control as well. A previous reported by Delgado and colleagues [26] demonstrated a greater reduction in HbA1c in the insulin Gla-100 group than regular insulin after 4-9 months of real-life treatment in Spain. Insulin-naïve patients showed similar results after six months of adding insulin Gla-100 to the maximum tolerated dose of oral hypoglycaemic drugs [27].As mentioned before, hypoglycaemia is a common complication of insulin therapy; both insulin and many of the oral drugs were reported to be associated with increased risk of hypoglycaemic events [28]. A previous meta-analysis study showed that insulin Gla-100 given once daily reduces the risk of hypoglycemia compared with regular insulin [29]. In addition, insulin Gla-100 was associated with low rate of hypoglycaemic events in the real-practice setting. Hanefeld et al. reported that only 2.45% of the poorly controlled T2DM patients experienced symptomatic hypoglycaemic events after six months of basal insulin treatment [30]. In the present study, 90 patients (37%) experienced hypoglycemic events. Only 6 of these symptomatic events were severe (2.5%); none of the hypoglycemic events was classified as serious.
5. Conclusions
- In conclusion, treatment initiation with insulin Gla-100 is an effective strategy for glycemic control in patients with T2DM poorly controlled on dual OAD treatments. Insulin Gla-100 effectively reduced HbA1c and improved glycemic control after six months of treatment under real-life conditions in Jordan. Insulin Gla-100 was well tolerated with a low incidence of adverse events including hypoglycemia. This simple regimen may facilitate effective insulin use in routine medical practice, improving achievement of recommended standards of diabetes care.
ACKNOWLEDGEMENTS
- The study was sponsored and funded by Sanofi, which played no role in analyzing or interpreting the data. A statistical analysis report was provided by RAY Contract Research Organization. Editorial support was provided by Dr. Ahmed El –Gebaly and Dr. Hussien Ahmed MD of RAY Contract Research Organization and was funded by Sanofi. All the patients were acknowledged of their participation in the study.
Disclosure
- R.N. is a speaker for Hikma, Dar Al Dawa, Pharma International, Merck & Co., Sanofi, Pfizer, Astra Zeneca, Novartis, Novo Nordisk, Menarini, Boehringer Ingelheim, Eli Lilly and Takeda. C. F., N. S., and J. T. are employees of Sanofi.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML