-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2019; 8(1): 9-16
doi:10.5923/j.diabetes.20190801.03

Nesfatin-1: a Potential Therapeutic Target in a Rat Model of Polycystic Ovary Syndrome
Mohamad Yosof Rezk1, 2, Hany Ahmed Elkatawy2, 3, 4, Rania A. Fouad4, 5, Eman T. Enan4, 6, Mohammed A. Attia4, 7
1Basic Medical Sciences dep. Unayzah College of Medicine and Medical Sciences, Qassim University, KSA
2Medical Physiology Department, Faculty of Medicine, Zagazig University, Zagazig, Egypt
3Obesity Management and Research Unit-Zagazig University Hospitals, Egypt
4College of Medicine, Almaarefa University, KSA
5Medical Biochemistry Department, Faculty of Medicine, Zagazig University, Zagazig, Egypt
6Department of Pathology, Faculty of Medicine, Mansoura University, Mansoura, Egypt
7Department of Pharmacology, Faculty of Medicine, Mansoura University, Mansoura, Egypt
Correspondence to: Mohamad Yosof Rezk, Basic Medical Sciences dep. Unayzah College of Medicine and Medical Sciences, Qassim University, KSA.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Polycystic ovary syndrome (PCOS) is a common endocrinopathy affecting 5-10 % of females of reproductive age. Based on the findings that PCOS is associated with obesity, metabolic disturbances and ovarian dysfunction and accounting for the observations that nesfatin-1 is an anorexigenic neuropeptide linked to insulin resistance and obesity, we postulated that nesfatin-1 administration may have beneficial effects for managing polycystic ovaries in rats. We aimed to investigate the effects of nesfatin-1 as a therapeutic approach in PCOS management. Materials and methods: 48 virgin female albino rats were divided into two main groups: Group I (Controls, n=24) and Group II (PCOS group, n=24) each group was divided into lean rats (group A) and obese rats (group B). Lean and obese rats in all the study groups either in control group or PCOS group were randomly subdivided into vehicle-treated group with 1 ml/kg/day of saline and nesfatin-1-treated group with a dose of 10 µg/kg/day of nesfatin-1. Body weight was recorded and food intake was calculated. Serum glucose, insulin, LH, FSH, Estradiol, Progesterone and Testosterone levels were measured. HOMA-IR was calculated. MalonylDiAldehyde (MDA), ovarian superoxide dismutase (SOD) and ovarian glutathione peroxidase (GPX) were measured. Histopathological examination was performed. Results: Nesfatin produced a significant inhibitory effect on body weight, food intake, glucose, insulin, HOMA-IR and LH level and a significant increase in FSH level. Also, nesfatin has a significant inhibitory effect on MDA and TNF-α and a significant stimulatory effect on SOX and GPx. Conclusion: our findings confirmed the potential therapeutic role of nesfatin in treatment of PCOS. Further studies should be conducted in human PCOS for application in clinical field.
Keywords: Nesfatin, Body weight, PCOS, Obesity, Estrogen, FSH, LH
Cite this paper: Mohamad Yosof Rezk, Hany Ahmed Elkatawy, Rania A. Fouad, Eman T. Enan, Mohammed A. Attia, Nesfatin-1: a Potential Therapeutic Target in a Rat Model of Polycystic Ovary Syndrome, International Journal of Diabetes Research, Vol. 8 No. 1, 2019, pp. 9-16. doi: 10.5923/j.diabetes.20190801.03.
1. Introduction
- Polycystic ovary syndrome (PCOS) is a common endocrinological disorder affecting 5-10 % of females of reproductive age [1]. The principle criteria of PCOS are hyperandrogenism, anovulation, infertility and ovarian follicular cysts. It is associated with cardiovascular disorders, obesity, insulin resistance, hyperlipidemia metabolic syndrome and type II diabetes [2]. The genetic and environmental aspects may have a role in the pathologenesis of PCOS [3]. Insulin resistance linked to abdominal obesity may result in ovarian and adrenal hyperandrogenism leading to adipose tissue malfunction and chronic inflammation triggering various metabolic and reproductive dysfunctions of PCOS [4, 5].Nesfatin-1, an 82-amino acid hypothalamic neuropeptide, is originating from nucleobindin-2 (NUCB2) [6, 7]. Specifically, it is secreted centrally from the hypothalamus and brain stem and peripherally from adipose tissue, gastric mucosa, pancreatic beta cells, and testis [8-10]. NUCB2/nesfatin-1 participates in regulating energy homeostasis, food intake, glucose metabolism, insulin sensitivity and reproductive functions. Centrally, nesfatin-1 inhibits food intake with subsequent chronic body weight reduction. In addition, it regulates insulin sensitivity by central action [11, 12]. Peripherally, it stimulates insulin release under hyperglycemic conditions [13], it can cross the brain-blood barrier in both directions [14]. Nesfatin-1 serum levels were found to be significantly decreased in the PCOS model rats in comparison with the normal control group [15]. Based on the findings that PCOS is associated with obesity, metabolic disturbances and ovarian dysfunction and accounting for the observations that nesfatin-1 is an anorexigenic neuropeptide associated with insulin resistance and obesity, we suggested that nesfatin-1 administration may have beneficial effects for managing polycystic ovaries in rats. We aimed to investigate the effect of nesfatin-1 as a therapeutic approach in PCOS management according to animal experiments.
2. Materials and Methods
- Experimental animals: This study was conducted on 48 young virgin healthy female albino rats, 6 weeks old with body weight 80-90 gm, were obtained from the animal house of faculty of veterinary medicine- Zagazig University. Rats were kept in steel wire cages (6/cage) in the physiology animal house in faculty of medicine -Zagazig University under hygienic conditions. Animals had free access to water, kept at room temperature and were maintained on a 12 h light/dark cycle. All rats received care in accordance with the national health guidelines and the study protocol was approved by the Institutional Review Board and ethics committee of faculty of medicine - Zagazig University.Animal grouping and induction of PCOS: Rats were divided into two main groups: Group I (Controls) (n=24) was divided into ordinary-diet fed (lean) rats (group IA) and the previously induced high-fat diet fed (obese) rats (group IB). All rats in group 1 were given 1 ml water orally by gavage daily for 21 days. The commercial rat chow consists of 25.8% protein, 62.8% carbohydrates and 11.4% fat [16]. However, for obesity induction, rats were fed on a high fat diet (protein 20%, carbohydrates 35% and fat 45%, mainly in form of lard and soy bean) for 9 weeks [17, 18]. Moreover, group II (PCOS group) (n=24) was divided into ordinary-diet fed (lean) rats (group IIA) and high-fat diet fed (obese) rats (group IIB). For PCOS induction, they were given oral administration of 0.5 mg/kg letrozole (non-steroidal aromatase inhibitor, ACDIMA international) dissolved in water daily for 21 consecutive days according to Kafali et al. [19]. Vaginal smears were obtained daily by vaginal washing with saline and fresh unstained samples were evaluated microscopically throughout the entire duration of the experiment, cycles with duration of 4 to 5 days were considered regular [19]. Estrus phases were determined according to Marcondes et al. [20] and Goldman et al. [21]. On the 22nd day of the experiment, and for 10 days, lean and obese rats in all the study groups either in control group or PCOS group were randomly subdivided into vehicle-treated group with 1 ml/kg/day of saline and nesfatin-1-treated group with a dose of 10 µg/kg/day of nesfatin-1. The dose of nesfatin-1 was used for therapeutic purposes according to the previous studies [22, 23].Anthropometric parameters measurement: the animals were weighed in (gm) by a digital scale; one day pre-experimental, twice weekly and on last day. The results were recorded for each labeled rat. Data were recorded for each labelled rat [24, 25].Blood sampling and biochemical analysis: At last day of experiment and after overnight fasting, rats were anaesthetized using ether (ADWIC Laboratory Chemicals, Egypt), blood samples were obtained from each rat sinus orbitus vein [26]. Controls sampling was taken in the estrus phase. Ovaries were dissected (right ovaries were immediately frozen at -70°C until used for determination of antioxidant markers whereas; the left ovaries of each group were immediately fixed in 4% paraformaldehyde for histopathology [27]. The blood samples were allowed to clot at room temperature before centrifuging at approximately 3000 rpm for 15 minutes (Centrifuge Zentriguanhan, Engelsr-of DDR- 7123. Engels-drf/-eipzig/Banug/Fabr.NO.08/30 type T30, Max. 1 min: 6400 Max Fullgew Kgr. Frequency 50 Hz made in Germany). The serum was stored at -20°C and examined for:- Measuring serum glucose levels (mg/dl) according to Tietz [28] and serum insulin levels (μIU/ml) by enzyme – linked immuno-sorbent assay (ELISA) according to Reaven [29]. Serum glucose and insulin kits were purchased from Biosource Europe S.A. Belgium.- Calculating homeostasis model assessment - insulin resistance (HOMA-IR) using HOMA-IR index according to Bonora et al. [30] - Measuring serum LH, FSH, Estrogen, Progesterone and Testosterone levels: according to Tietz, [28] using rat commercial kits purchased from Sigma Co.- Measuring ovarian lipid peroxidation (MDA) as described by Ohkawa et al. [31], ovarian superoxide dismutase (SOD) according to Kakkar et al. [32] and ovarian glutathione peroxidase (GPX) according to Reddy et al. [33]Histopathological examinationDissection of ovaries was performed and fixed in 10% formalin for 6 hours at room temperature then washed in a phosphate buffer saline solution. For light microscopical examination, dehydration of fixed tissues was done by using ascending series of ethanol, cleared in xylene and embedded in paraffin. 5 m thick sections were mounted in slides previously treated with 3- aminopyropyltriethoxysilane and stained with hematoxylin-eoisin preliminary observation [27].Statistical Analysis: The data obtained in the present study were expressed as mean ±SD. Unpaired T test was done for comparing means. The statistical analysis is done by using Graphpad Quickcalc internet site. P value < 0.05 was considered statistically significant.
3. Results
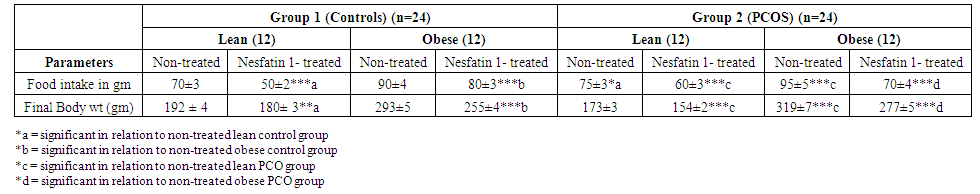 | Table 1. Effects of Nesfatin-1 on food intake and Body weight in controls and PCOS rats |
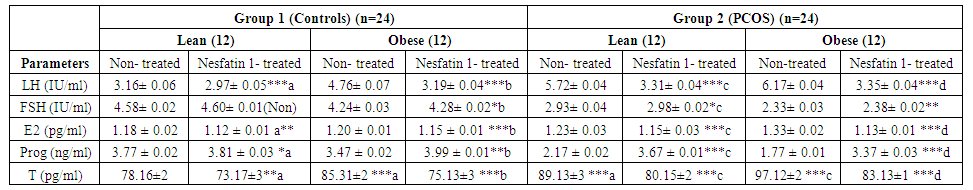 | Table 2. Effects of Nesfatin-1 on sex hormones in control and PCOS rats |
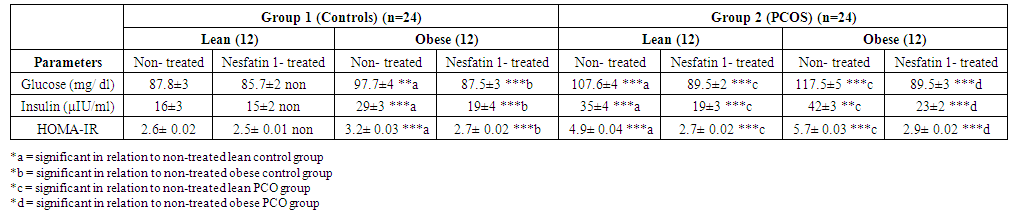 | Table 3. Effects of Nesfatin-1 on glucostatic parameters in controls and PCOS rats |
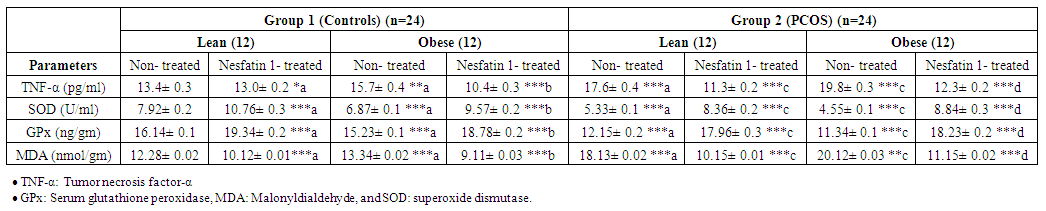 | Table 4. Effects of Nesfatin-1 on TNF-α and antioxidants in controls and PCOS rats |
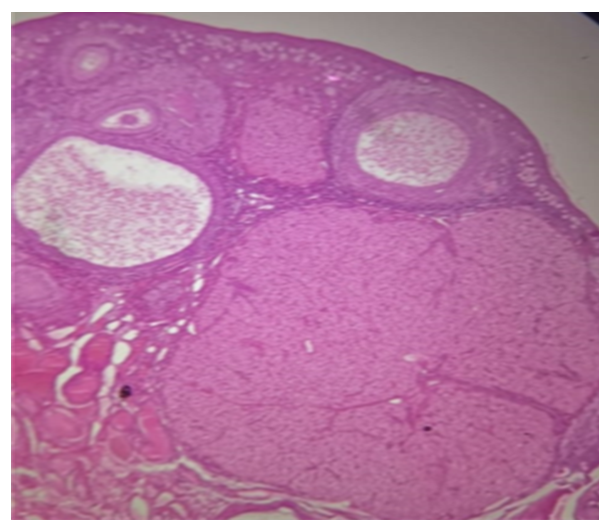 | Figure 1. Section of the ovary in control group follicles at various stages of development including secondary follicles, Graafian follicles, and corpus luteum (H&E, x40) |
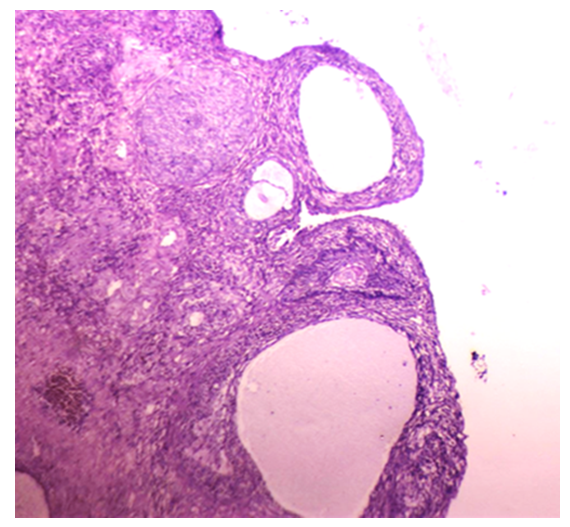 | Figure 2. Sections of the ovary in group IIB (obese PCOS): ovarian cortex shows multiple cystic follicles, with diminished granulosa cells and thickened leutinized theca cell layer (H&E, x40) |
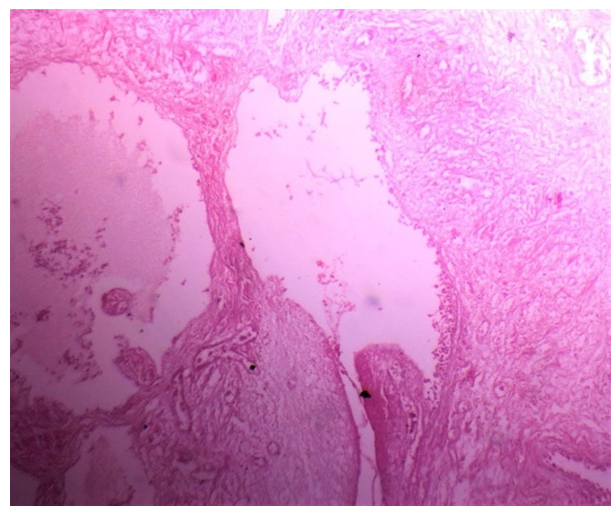 | Figure 3. Section of ovary in group IIB (obese PCOS) large cystic follicles,fewer layers of granulosa cells, and heavily collagenized ovarian stroma (H&E, x40) |
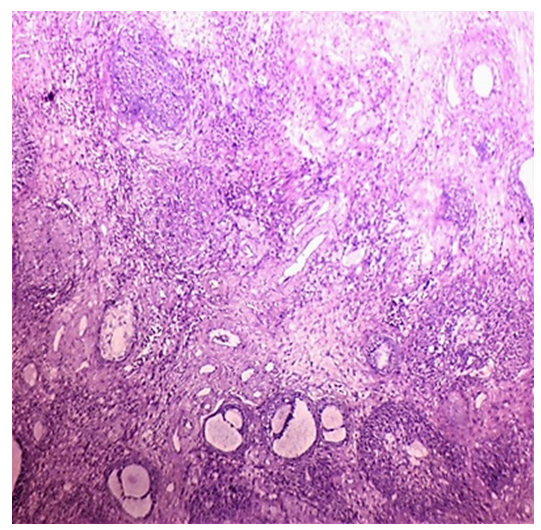 | Figure 4. Sections of the ovary in group IIA (lean PCOS):increase in numbers of atretic follicles, and stromal hyperthecosis (H&E, x40) |
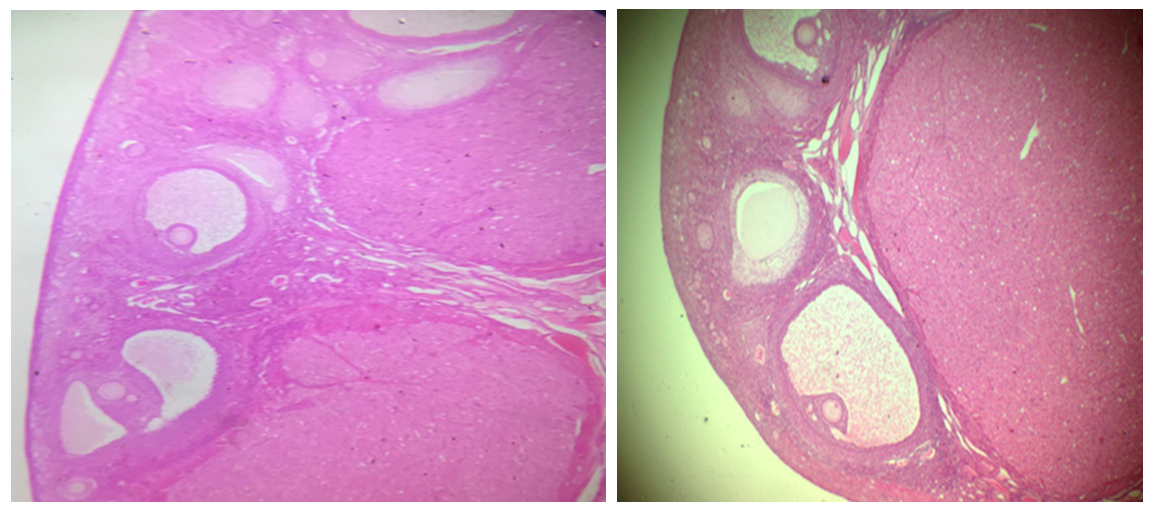 | Figure 5. Sections of ovary of the nesfatin-1-treated groups showing increased number of antral follicles and corpus luteum with thick granulosa cell layer (H&E, x40) |
4. Discussion
- Polycystic ovary syndrome (PCOS) is a common endocrinological disorder characterized by hyperandrogenism, anovulation, infertility and ovarian follicular cysts. It is associated with cardiovascular disorders, obesity, insulin resistance, hyperlipidemia metabolic syndrome and type II diabetes [2].Nesfatin-1 serum levels were found to be significantly decreased in the PCOS model rats in comparison with the normal control group [15]. Based on the findings that PCOS is associated with obesity, metabolic disturbances and ovarian dysfunction and accounting for the observations that nesfatin-1 is an anorexigenic neuropeptide linked to insulin resistance and obesity, we hypothesized that nesfatin-1 administration may have beneficial effects for managing polycystic ovaries in rats. Aim of this study is to demonstrate the effect of nesfatin-1 as a therapeutic approach in PCOS management. In this study, nesfatin-1 reduced food intake significantly in lean rats and in obese rats (p≤0.0001). Nesfatin-1 also significantly reduced food intake in PCO lean and obese rats (p≤0.0001). Nesfatin-1 produced a significant inhibitory effect on body weight in all groups (p≤0.0001). The results of this study were supported by Deniz et al., 2012, Alp et al., (2015) who reported that BMI was negatively correlated with nesfatin-1 levels among PCOS subjects [34, 35]. Also, we are in agreement with Panidis et al., 2013, Barber et al., 2016) who found that nesfatin-1 level was negatively correlated with body weight and they showed that weight loss effectively restore ovulation and decrease insulin and testosterone levels in women with PCOS [36, 37].In the present study, nesfatin-1 produced highly significant inhibitory effect on LH levels in all groups (p≤0.0001). This finding was supported by Xu et al., (2017) [38] who found that nesfatin-1 serum levels decreased significantly in the PCOS model rats compared with the normal control group. However, the findings of this study were in controversy with (Garcia-Galiano et al. 2010) [39] who found that 50 pmol nesfatin-1 i.c.v. did not affect plasma LH in adult female rats. Our results were in partial agreement with Tadross et al. 2010 [40], Patterson et al. 2011) [41] who indicated that upon i.c.v. administration of a very high dose of nesfatin-1 (1 nmol), plasma LH and FSH were increased in male rats. We found that LH levels decreased in controversy with them. However, the findings of the present study were supported by (Gao et al. 2016) [42] who found that i.c.v. administration of nesfatin-1 significantly reduced the expression of GnRH mRNA in the hypothalamus and FSH and LH mRNA in the pituitary, suggesting an inhibitory role of nesfatin-1 on the HPG axis. We were also in agreement with Gonzalez et al. 2012) [43] who found that a single intraperitonial injection of synthetic nesfatin-1 (50 ng/g b. w.) produced a significant reduction (∼60%) in serum LH levels at 60 min post-administration. Nesfatin-1 produced non-significant effect on FSH levels in lean control group however Nesfatin-1 increased FSH levels significantly in other groups (p≤0.05) in control obese and lean PCO groups and highly significant effect in PCO obese group (p≤0.005). These findings are in agreement with Xu et al., (2017) [38] who found a positive correlation between nesfatin-1 and FSH serum level (p≤.05) and they suspected that, in PCOS, the decrease in nesfatin-1 can arrest follicular cells through the inhibition of FSH in folliculogenesis. Estrogen significantly decreased in lean nesfatin-treated group in comparison with lean control group (p≤0.05). Nesfatin-1 produced highly significant reductions in estrogen level in all other groups (p≤0.0001). These findings are in agreement with (Garces et al. 2014) who found that Nucb2 mRNA expression is regulated by 17-estradiol and progesterone sex steroids and they suggested an existence of a feedback-mechanism in the NUCB2/nesfatin-1–HPG-axis interaction [44].Progesterone significantly increased in nesfatin group in comparison with non-treated group (p≤0.05). Nesfatin-1 produced highly significant increase in progesterone level in obese control and obese PCO groups (p≤ 0.005 and p≤0.0001). Nesfatin-1 decreased testosterone level significantly in all groups (p≤0.0001). These findings are in agreement with Xu et al., (2017) who reported that nesfatin-1 may play an inhibitory role in the conditions of hyperandrogenism and obesity that accompany and define PCOS. They concluded that a decrease in both nesfatin-1 and its mRNA in ovarian tissues might promote the development of PCOS [38].In the present study, we found that glucose level is significantly high (p≤0.0001) in PCO groups compared with control groups and in obese groups relative to lean groups. In addition, we found that insulin level and HOMA-IR are significantly (p≤0.0001) higher in PCO groups compared with control groups. These findings are supported by Deniz et al., 2012) who reported that PCOS patients had significant blood glucose, gonadotropin and androgen plasma levels and HOMA-IR compared with normal control women [34].In our study, nesfatin-1 produced non-significant reduction in glucose level in lean control group however it produced significant reductions in obese control, lean PCO and obese PCO groups (p≤0.0001). These findings are supported by Su et al., 2010) who reported a dose-dependent reduction of blood glucose following intravenous injection of nesfatin-1 under conditions of hyperglycemia as observed in leptin receptor deficient db/db mice, whereas no effects were observed in normoglycemic C57BLKS/J mice [45]. Nesfatin-1 also produced non-significant reduction in insulin level in lean control group however it produced significant reductions in obese control, lean PCO and obese PCO groups (p≤0.0001). HOMA-IR was decreased significantly in all groups (p≤0.0001) except lean control group. Glucose, Insulin and HOMA-IR were increased significantly in obese control and lean PCO groups in relation to lean control group and also in obese PCO group in relation to lean PCO group (p≤0.0001). Deniz et al., 2012) who found a negative correlation between nesfatin-1 and BMI, glucose, insulin levels and a HOMA-IR in PCOS subjects. They concluded that lower nesfatin-1 level might have an important role in development of PCOS [34]. In this study, nesfatin-1 significantly decreased tumor necrosis factor-α (TNF-α) and MDA in all groups (p≤0.0001) in relation to control. Also, nesfatin-1 significantly increased SOD and GPx in nesfatin treated group in relation to non-treated subgroups (p≤0.0001) in obese and lean groups. These findings are supported by Solmaz et al., 2016) who found a significant reduction (p = 0.032) in MDA levels and non-significant reduction in interleukin-6 and TNF-α in nesfatin treated group compared with the control group [46].In the present study, Histological analysis of ovarian sections in Nesfatin-1-treated PCOS group, showed diminished number and size of cysts in the ovarian cortex. In addition, increased number of antral follicles and corpora lutea with thick granulosa cell layer in contrast to PCOs-only groups. Moreover, stromal cortical sclerosis and hyperthecosis were less evident (Figure 5 and 6).In conclusion, nesfatin-1 produced a significant inhibitory effect on body weight, food intake, glucose, insulin, HOMA-IR and LH level and a significant increase in FSH level. Also, nesfatin has a significant inhibitory effect on MDA and TNF-α and a significant stimulatory effect on SOX and GPx. These findings confirmed the potential therapeutic role of nesfatin in treatment of PCOS. Further studies are needed in human PCOS for application in clinical field.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML