-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2019; 8(1): 1-3
doi:10.5923/j.diabetes.20190801.01

Screening for ICA Autoantibodies among Healthy Young Adults from Bethlehem District: A Pilot Study
Nader Hazboun, Shirin Sayed Ahmad
Biology Department, Bethlehem University, Bethlehem, Palestine
Correspondence to: Nader Hazboun, Biology Department, Bethlehem University, Bethlehem, Palestine.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Diabetes mellitus (DM) is a common disorder in the Palestinian population. It causes economic burden on the society due to costs of management of the disease. Therefore, being able to predict the development of type 1 DM in young adults by screening for islet cell (ICA) antibodies before its onset by many years and reverse the process by instituting proper measures would have a tremendous impact on the individual and population levels. This pilot study involved screening 99 healthy young adults with no history of DM in Bethlehem District and a mean age of 28.9 years, (range 19-40 years) for ICA autoantibodies. All samples were normoglycemic. Of the 99 samples screened, 5.1% tested positive for ICA antibodies. This 5.1% frequency of ICA antibodies among healthy adults in Bethlehem District indicates that type 1 DM might be on the rise in the future so that larger studies are recommended. At high-risk patients who tested positive for ICA antibodies are recommended to regularly monitor their blood sugar levels in the coming years for earlier detection of type 1 diabetes.
Keywords: Type 1 diabetes, Islet cell antibodies
Cite this paper: Nader Hazboun, Shirin Sayed Ahmad, Screening for ICA Autoantibodies among Healthy Young Adults from Bethlehem District: A Pilot Study, International Journal of Diabetes Research, Vol. 8 No. 1, 2019, pp. 1-3. doi: 10.5923/j.diabetes.20190801.01.
Article Outline
1. Introduction
- Type 1 diabetes, also known as insulin-dependent diabetes mellitus (IDDM), results from the simultaneous action of specific auto-reactive CD4+ and CD8+ T lymphocytes which destroy the insulin-secreting pancreatic beta cells. Autoantibodies against different antigens of the islet cells can be detected in the serum of type 1 DM patients many years before overt diabetes. These autoantibodies are used as important biomarkers to identify persons with increased risk to develop diabetes at a time when all metabolic tests available still show normal results. Islet cell autoantibodies (ICA) have been the first serological markers described for type 1 diabetes mellitus. These antibodies belong to the immunoglobulin G (IgG) subtype and are directed against a variety of islet cell antigens. Many studies across the globe were done on serum ICA antibodies as a predictive marker for type 1 DM. The best performing combination was ICA, plus islet antigen 2 (IA-2A) showing 52.6% sensitivity, 99.8% specificity, 51.3% positive predictive value (PPV) and 99.8% negative predictive value (NPV) [1]. The data offer a solid rationale for future testing of ICA and IA-2A as routine laboratory markers to identify individuals at high risk of T1DM in the general population [1]. Positivity for multiple (≥2) autoantibodies is highly predictive of clinical disease both among first-degree relatives and in the general population. The first signs of beta-cell autoimmunity may appear early during the first months of life. The majority of those individuals diagnosed with T1D before puberty seroconvert to autoantibody positivity before the age of 3 years. The natural course and duration of preclinical diabetes vary substantially from one individual to another [2]. Islet autoantibodies are markers of type 1 diabetes and an increase in number of autoantibodies detected during the preclinical phase is predictive of progression to overt disease. After median follow-up of 2 years, 141 relatives had developed ≥ 1 additional autoantibodies. In relatives with insulin antibodies (IAA), spread of islet autoimmunity is largely limited to early childhood, while immune responses initially directed at glutamic acid decarboxylase (GAD) can mature over a longer period of time. These differences have important implications for monitoring these subjects and for designing prevention trials [3]. This pilot study involved screening for ICA antibodies among young healthy male and female adults in Bethlehem District with no history of DM, a mean age of 28.9 years (range 19-40 years) and normal fasting blood sugar levels.
2. Materials and Methods
- This pilot study involved screening 99 serum samples for ICA antibodies from healthy young male and female adults with no history of Diabetes, a mean age of 28.9 years (range 19-40 years) and with normal fasting blood sugar (FBS) levels from Bethlehem district using the ICA Enzyme linked immunosorbent assay (ELISA) kit from CUSABIO Biotech company. All participants were Bethlehem citizens. Whole blood samples were collected in serum tubes in a 10-month duration starting from February 2018 till November 2018. All patients gave their informed consent prior to analysis. The method used in this study employs the qualitative enzyme immunoassay technique which measures the presence or absence of ICA antibodies. The microtiter plate is pre-coated with antigen. Samples are pipetted into the wells with anti-human IgG conjugated Horseradish Peroxidase (HRP). Any present antibodies specific for the antigen will bind to the pre-coated antigen. Following a wash to remove any unbound reagent, a substrate solution is added to the wells and color develops in proportion to the amount of islet cell antibody (ICA) bound in the initial step. The color development is stopped and the optical density (OD) of each well is measured using a microplate reader set to 450 nm. For calculation, the sample OD is compared with the control OD; if OD sample/OD negative ≥2.1 the sample is considered positive, but if the OD sample/OD negative <2.1 it is considered negative [4].
3. Results
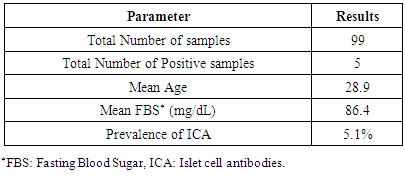
- All 99 serum samples that were screened for ICA antibodies have normal FBS levels. Of the 99 samples screened, five tested positive resulting in a 5.1% frequency of ICA among young healthy adults in the population of Bethlehem District (Table 1).
|
4. Discussion
- Autoimmune diabetes is a heterogeneous disease that is generally regarded as a condition that presents in childhood or adolescence. However, a substantial proportion of patients experience onset in adulthood. In this case, termed adult-onset autoimmune diabetes, the disease is even more heterogeneous than young-onset autoimmune diabetes, as the rate of βcell destruction is highly variable, which is probably due to differences in the penetrance of genetic and immune factors [5]. In 2016, the estimated prevalence of diagnosed type 1 diabetes in USA was 0.55% [6]. Islet autoantibodies are thought to be an epiphenomenon rather than key pathogenic factors in islet-cell destruction; however they are used to discriminate autoimmune from non-immune diabetes [5]. Most studies relied on measuring glutamic acid decarboxylase antibody (GADA), islet cell antibody (ICA), or insulin-associated antibodies (IAA) for diagnosing or predicting type 1 diabetes. For example in Iran, a population-based study of GADA involving 500 patients found the frequency of autoantibodies to be at 14.2% [7]. In this study we screened for ICA antibodies among healthy young male and female adults with no history of DM and a mean age of 28.9 years (range 19-40 years). This biomarker is known as being predictive for type 1 DM [1], so the 5.1% of individuals who tested positive for ICA antibodies are recommended to monitor their fasting blood sugar levels on a regular basis in the coming years so that type 1 DM would be detected earlier and proper action instituted.
5. Conclusions
- This 5.1% frequency of ICA antibodies in Bethlehem District among healthy male and female young adults indicates that type 1 DM might be on the rise in the future so that larger studies are recommended. Screening programs designed for populations at risk should enable earlier intervention with improved metabolic outcomes, appropriate therapy and improved identification of comorbidities [7].
ACKNOWLEDGEMENTS
- We thank Bethlehem University for funding this study through an internal research grant. We would also like to thank all those who have provided us with the plasma/serum samples in this pilot study especially Tafish Medical Laboratory, Odeh Medical Laboratory, and Medicare Labs in Bethlehem.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML