-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2018; 7(3): 57-62
doi:10.5923/j.diabetes.20180703.03

Serum Level of Progranulin and Microvascular Complication in Type 2 Diabetes
Doaa SE Zaky1, Kareema Y. Ahmed1, Samiha A. Y. Abd-Rabo1, Nagwa A. G. Mohammed2, Mayada F. S. Osman3
1Department of Internal Medicine, Al-Zahraa University Hospital, Al-Azhar University, Cairo, Egypt
2Department of Clinical and Chemical Pathology, National Research Center, Cairo, Egypt
3Department of Internal Medicine, Materia Teaching Hospital, Cairo, Egypt
Correspondence to: Doaa SE Zaky, Department of Internal Medicine, Al-Zahraa University Hospital, Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: As the seventh leading cause of death by 2030, diabetes is considered the most common chronic metabolic disease worldwide. Progranulin (PGRN) is a novel protein that involved in chronic inflammatory response and insulin signalling pathway in obesity, metabolic syndrome and Type 2 diabetes millets (T2DM). Objectives: This study aimed to investigate the relation between PRGN and diabetic microvascular complications (nephropathy and retinopathy). Methods: A prospective cross sectional observational study was carried out on 60 T2DM patients with and without microangiopathies and 20 age and sex matched healthy controls. PGRN serum levels and glucose metabolism related laboratory data were measured and recorded. Results: Serum levels of PGRN were significantly higher in T2DM patients compared to normal control and in patients with microangiopathies compared to patients without microangiopathies (simple diabetes millets (SDM)). Serum PGRN levels were positively correlated with duration of diabetes, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), triglyceride (TG), low density lipoprotein (LDL), albumin/creatinine ratio (A/C ratio), Glycosylated haemoglobin (HbA1c), fasting blood sugar (FBS), fasting insulin (FINS), Homeostasis Model Assessment for Insulin Resistance (HOMA-IR), while correlated negatively with serum albumin and high density lipoprotein (HDL). PGRN serum levels increased with the progress of diabetic microangiopathies with significantly highest values detectable in advanced nephropathy and retinopathy. Conclusion: PGRN seems to be involved in the pathogenesis of diabetic microangiopathy and might be considered as a biomarker for its severity in T2DM.
Keywords: Progranulin, Microangiopathy, Retinopathy, Nephropathy
Cite this paper: Doaa SE Zaky, Kareema Y. Ahmed, Samiha A. Y. Abd-Rabo, Nagwa A. G. Mohammed, Mayada F. S. Osman, Serum Level of Progranulin and Microvascular Complication in Type 2 Diabetes, International Journal of Diabetes Research, Vol. 7 No. 3, 2018, pp. 57-62. doi: 10.5923/j.diabetes.20180703.03.
Article Outline
1. Introduction
- T2DM is the most common type of diabetes, accounting for around 90% of all cases. Its prevalence is rapidly emerging worldwide since 1980 [1]. There are more than 500 million prevalent cases of T2DM worldwide in 2018 and the prevalence expected to increase in the next 10 years, but greatest growth will be experienced in lower-income countries [2]. T2DM is most commonly seen in older adults, but it is increasingly seen in children, adolescents and younger adults due to the increasing prevalence of obesity [3]. Obesity is associated with a low-grade inflammation of white adipose tissue resulting from chronic activation of the innate immune system that can subsequently lead to insulin resistance, impaired glucose tolerance and diabetes [4, 5]. Activation of inflammatory processes may also contribute to vascular damage that promote long-term complications of diabetes [5, 6]. Diabetic microangiopathy is a typical diabetic complication that clinically reflected by diabetic nephropathy, diabetic retinopathy, and diabetic peripheral neuropathy. Approaches to the treatment of diabetic complications in the future may involve regulation of inflammatory processes by targeting factors that contribute to vascular damage. Progranulin (PRGN) is a 68–88 kDa protein which is widely expressed in body tissues including adipose tissue. Full length PGRN promotes cell growth and survival and has an anti-inflammatory activity. However, PGRN is rapidly cleaved in tissues by elastase and proteinase 3 to generates granulin peptides that promote inflammation [7]. It was reported that circulating PGRN levels are elevated in patients with obesity [8], insulin resistance [9], metabolic syndrome, and T2DM [10]. In this context, we design a cross sectional observational study that aiming to outline the role of PGRN in the pathogenesis of diabetic microangiopathy as this relationship is still not fully investigated.
2. Subjects and Methods
2.1. Study Participant
- This is a prospective cross sectional observational study. The subjects included were 60 patients with T2DM, diagnosed according to WHO criteria 1999 [11], and 20 age and sex matched healthy controls. The patients were recruited from in-patient department and out-patient clinic of Mataria hospital, Cairo, Egypt. Informed consent was obtained from all study participants in advance. All procedures were performed in accordance with the guidelines in the Declaration of Helsinki and approved by Research Ethics Committee of Faculty of Medicine for Girls, Al-Azhar University. Exclusion criteria were diabetic macrovascular complications, other endocrine diseases that affect glucose or lipid metabolism, chronic hepatitis, primary kidney disease, acute recent inflammatory diseases, degenerative nervous system diseases, and malignancy. All patients were subjected to a standardized questionnaire including: age, sex, duration of T2DM, comorbidity and medications received. Physical examination was done with special emphasis to resting blood pressure, height, weight. Body mass index (BMI) was calculated as weight/height 2 (kg/m2).
2.2. Laboratory Investigations
- All measurements were taken in the morning after 12 hours overnight fasting. Complete blood picture (CBC) was performed on Coulter Counter T890 (Coulter Counter, Harpenden, UK). Kidney functions, fasting blood sugar (FBS) and lipid profile (cholesterol, triglyceride, LDL, HDL) were carried out on Dimension RxL Max analyzer (Siemens Healthcare GmbH - Henkestr. 127, 91052 Erlangen, Germany) by colorimetric techniques. Glycosylated haemoglobin (HbA1C) was performed by cation exchange resin. Fasting serum insulin was determined using radioimmune assay. The Homeostasis Model Assessment for Insulin Resistance (HOMA-IR) was calculated using the following equation: HOMA-IR = fasting blood glucose (mg/dl) x fasting serum insulin (mU/l)/405 [12]. Albumin concentrations were measured in urine using a Minineph microalbumin kit based on nephlometry method on Minineph-nephelometer (AD200) (The Binding Site, Birmingham, UK) [13].Urinary creatinine was determined on Dimension RxL Max analyzer by colorimetric technique and the ratio of urine albumin to creatinine was used to define microalbuminuria. Estimated glomerular filtration rate (eGFR) was calculated by Cockcroft–Gault (CG) Formula calculation [14]. Creatinine clearance (ml/minute) = [(140-age) x weight)/72 x serum creatinine] (x0.85 in female). Progranulin was determined using quantitative sandwich enzyme immunoassay technique supplied R & D Systems Europe, Ltd. (19 Barton Lane, Abingdon Science Park, Abingdon OX14 3NB, UK).
2.3. Imaging Study
- Fundoscopic examination was performed by a professional ophthalmologist using direct ophthalmoscopy after pupillary dilatation to diagnose diabetic retinopathy. Electrocardiogram (ECG) and Doppler studies of the lower limb vascular system were carried on rolling out diabetic macrovascular complications [15].
2.4. Statistical Analysis
- Data were collected, revised, coded and entered to the Statistical Package for Social Science (IBM SPSS) version 23. Data was described in terms of mean ± standard deviation (±SD) for continuous variables and number with percentage for categorical variables. Student’s T test and one-way analysis of variance (ANOVA) were used to compare the differences among the groups. Chi square-test was used for comparison between 2 independent groups or to study the association between each 2 variables as regard to categorized data. Ranked Spearman correlation coefficients were used to assess possible association between variables for non-parametric data. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the probability of error at (0.05) was considered significant and at 0.01 and 0.001 were highly significant.
3. Results
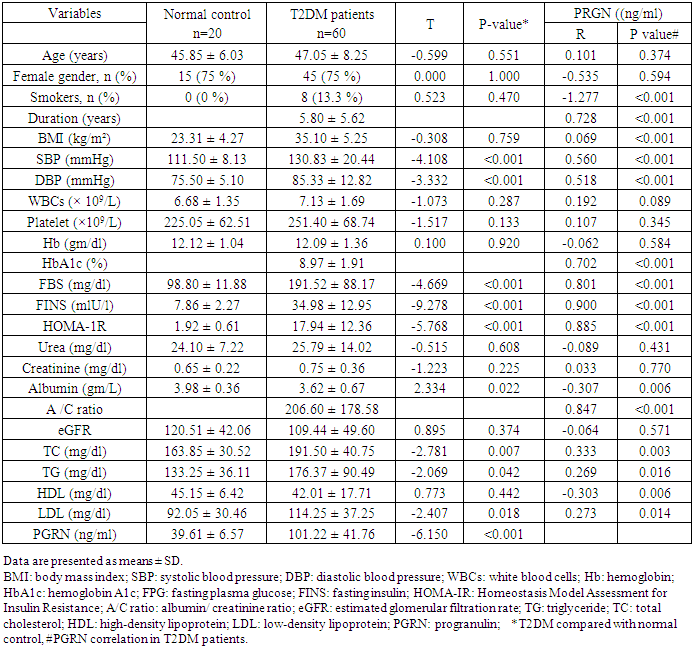
- Clinical and biochemical characteristics of 60 patients with T2DM and 20 normal controls were summarized in table (1). There were no statistical differences as regard to age and sex between both groups, however, BMI was significantly increased in T2DM compared to normal controls. The circulating level of PGRN was significantly increased in patients with T2DM compared with normal control group. The circulating PGRN level in T2DM patients showed significant positive correlation with duration of T2DM, SBP and DBP (P <0.001). As regard to lipid profile; PRGN showed significant positive correlation to TC, TG, LDL and negative correlation with HDL (P <0.05). Circulating PGRN level was also positively correlated to glucose parameters (FBS and HbA1c) as well as FINS and insulin resistance in T2DM patients. As regard to kidney functions, PGRN level was negatively correlated to serum albumin and positively correlated to albumin/ creatinine ratio; however, no correlation was detected with urea, creatinine or eGFR.
|
|
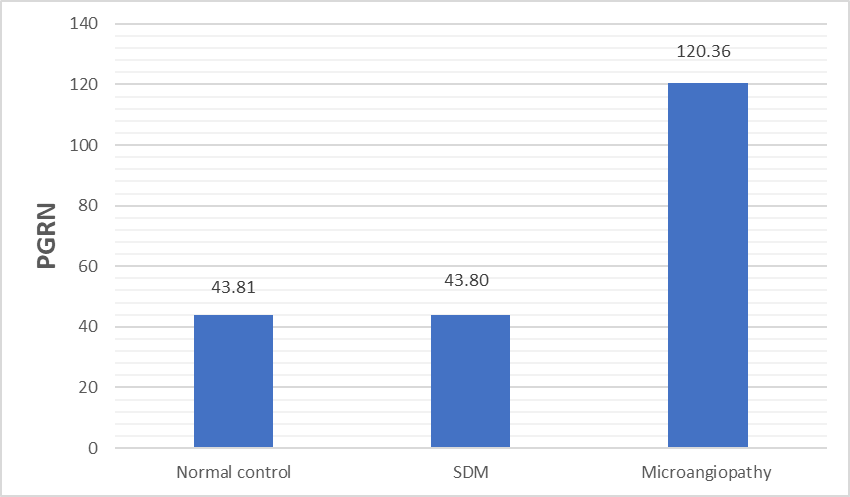 | Figure 1. PGRN serum levels in normal controls and T2DM with or without microangiopathies. SDM: simple diabetes millets; PGRN: progranulin µg/ml |
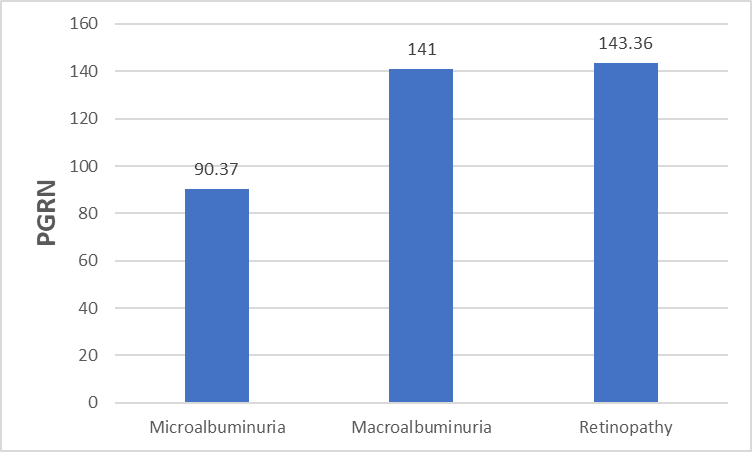 | Figure 2. PGRN serum levels in T2DM patients with microalbuminuria, macroalbuminuria and retinopathy. PGRN: progranulin (µg/ml) |
4. Discussion
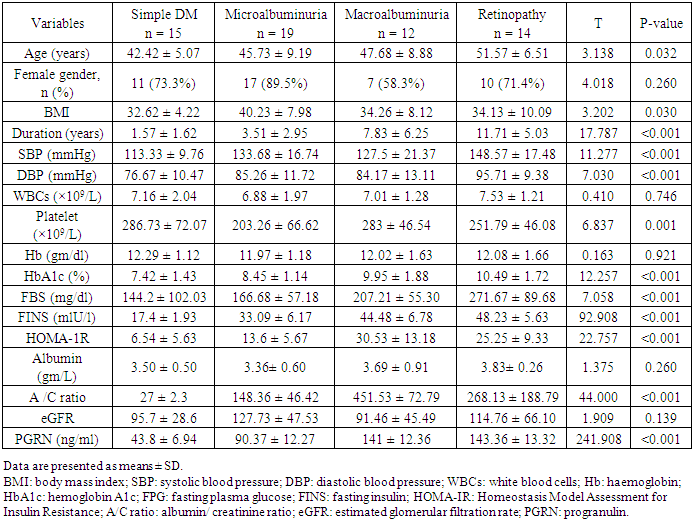
- Type 2 diabetes millets is the most common type of diabetes, accounting for around 90 % of all cases, and its rapid emergence worldwide has led to its classification as an epidemic [16, 17]. Chronic complications are the major outcome of T2DM which incur heavy burdens to the patients and health care system [18]. PGRN is a multifunctional protein, which has been implicated in cell growth, wound repair and inflammation [19]. Studies in the last decade have shown that inflammation is a key process in the development of DM and its complications [5, 6]. In this context, we investigated the role of PGRN in the development of microangiopathy in T2DM. The present study demonstrated that PGRN concentration was markedly elevated in sera of patients with T2DM as compared to normal controls and in patients with microangiopathies as compared to SDM. Moreover, we did not find significant difference between PGRN levels in normal control and SDM. These observations seem to suggest that PGRN may be involved in the pathogenesis of diabetic microangiopathy. Our results were augmented by Lin Xu et al [20] study that demonstrated marked increase in circulating PGRN concentrations and other inflammatory markers (TNF-𝛼 and IL-6) in T2DM patients with microangiopathy with remarkable positive correlation between serum PGRN levels and those inflammatory markers.PGRN could stimulate the adipocytes to release more IL-6 as the increased secretion of IL-6 (by TNF-𝛼) was completely blocked by ablation of PGRN gene in 3T3-L1 adipose cells [21]. Taking these results into consideration, PRGR is closely related to the proinflammatory state frequently seen in diabetic microangiopathy and may be considered as a biomarker for the chronic inflammatory response in diabetic microangiopathy. The interactions between progranulin and inflammation can be explained by the physiological function of PGRN, with the full-length form having anti-inflammatory activity, whereas proteolytic cleavage generates granulin peptides that promote inflammatory activity and neutralize the anti-inflammatory effect of intact progranulin [22, 23].Our results were concordant with other studies [24, 25] that reported positive correlation between PGRN and parameter of glucose metabolism namely Hb A1C, fasting plasma glucose in DM patients. Other studies demonstrate a positive association between circulating PGRN and components of the metabolic syndrome including insulin resistance, hypertension, and dyslipidemia [24, 26]. In agreement with these previous studies, our results demonstrated positive correlation between circulating PRGN with SBP, DBP, FINS, HOMA-1R, TC, TG, LDL and negative correlation with HDL. PGRN can affect lipid metabolism by inhibiting free fatty acid uptake and lipogenesis and/or by enhancing intracellular lipolysis [27].A linear relationship between microvascular complications and duration of DM was established (Chawla A, et al 2014) [28]. Chronicity of hyperglycemia is another mechanism for development of microvascular and macrovascular complication in T2DM (Aastha Chawla et al 2016) [29]. The present study reported significant positive correlation of PRGN with duration of DM and the level of Hb A1c. To further clarify the relationship between the increased PGRN and the development of diabetic microangiopathies, we tested PGRN levels in patients with different stages of diabetic nephropathy and patients with diabetic retinopathy; PGRN levels was significantly increased in group of advanced nephropathy stage (macroalbuminuric group) and in group of retinopathy (P <0.001). Like our study findings, a study has reported PGRN serum level increased with the progress of diabetic microangiopathies significantly highest values detectable in clinical diabetic nephropathy and proliferative diabetic retinopathy groups [20].PGRN serum levels were reported to increase with deteriorating renal function, and the renal elimination was a major route for circulation PGRN [30]. Our study demonstrated no significant difference in eGFR between different groups of microangiopathy and no significant correlation were found between PGRN and eGFR, urea or creatinine. However, in another study [20], PGRN was independently correlated with serum urea and creatinine. Further studies were needed to determine whether the increased RGRN level in diabetic microangiopathy due to increased production from adipose tissue or decreased elimination secondary to renal impairment. Limitations in our study include small sample size and the study design that does not clarify the causal relationship between serum progranulin levels and the presence of diabetic microangiopathy.
5. Conclusions
- Circulating serum PGRN concentration was significantly increased in T2DM patients compared to age and sex matched healthy controls and in T2DM patients with microvascular complications compared to simple T2DM patients. The increased PGRN serum levels were closely related to the progress of diabetic microangiopathy, suggesting that PGRN may be considered as a marker for diabetic microangiopathy and its severity. The level of PGRN in patients with T2DM could be a potential therapeutic target for the management of diabetic microangiopathy. Further studies using larger populations will be needed to confirm our observations and to validate the current findings.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML