-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2018; 7(3): 41-49
doi:10.5923/j.diabetes.20180703.01

Study of Pentraxin-3 as an Early Marker of Diabetic Nephropathy in Type 2 Diabetes Mellitus
Fatma M. El-Senosy1, Mervat Elshahat Elwakeel2, Rasha Elsayed Mohamed3
1Departments of Internal Medicine, Faculty of Medicine (Girls) Al-Azhar University, Cairo, Egypt
2Departments of Endocrinology, Faculty of Medicine (Girls) Al-Azhar University, Cairo, Egypt
3Departments of Medical Biochemistry, Faculty of Medicine (Girls) Al-Azhar University, Cairo, Egypt
Correspondence to: Fatma M. El-Senosy, Departments of Internal Medicine, Faculty of Medicine (Girls) Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Diabetic nephropathy is the most common microvascular complication of diabetes mellitus. Underlying mechanisms of diabetic nephropathy are related to various metabolic and inflammatory pathways. Pentraxins are super family of acute-phase proteins that induce short Pentraxins such as C-reactive protein (CRP) or long Pentraxins such as pentraxin-3. PTX3 is considered to be a candidate of vascular inflammation marker to evaluate vascular complications of diabetes mellitus. Objective: This work was carried out to evaluate serum level of Pentraxin-3 (PTX3) in patients with type 2 diabetes mellitus and to assess its correlation with high sensitivity C reactive protein (hs-CRP) and urinary albumin excretion as predictor markers for early diabetic nephrology. Patients and methods: A case control study was conducted on 60 patients with type 2 diabetes mellitus (group 1) and 30 age matched healthy controls (group 11). Group 1 was divided according to urinary albumin excretion into 2 subgroups: group (1a): 30 diabetic patients with normo albuminuria and group (1b): 30 diabetic patients with micro albuminuria. Routine laboratory investigations, fasting blood sugar, post prandial blood sugar, HbA1c, lipid profiles, serum pentraxin-3, serum hs-CRP were done for all studied groups and A24 h urinary albumin excretion was done for patients only. Results: There were highly significant Elevation of serum pentraxin-3 and hs-CRP in Group 1 when compared to group 11 and These elevations were more significant in (group 1b) when compared to (group 1a) and There was significant positive correlation between serum pentraxin-3 and each of serum hs-CRP, HbA1c, and duration of diabetes and there was highly significant positive correlation between pentraxin-3 and urinary albumin excretion in group 1b. Conclusion: The study concluded that a highly significant elevation of serum pentraxin-3 and hs-CRP in Egyptian diabetic patients and there was a highly significant positive correlation between pentraxin-3 and urinary albumin excretion. We recommend that measurement of serum pentraxin-3 and serum hs-CRP as screening method for early detection of diabetic nephropathy.
Keywords: Diabetic nephropathy, Pentraxin-3, hs-CRP
Cite this paper: Fatma M. El-Senosy, Mervat Elshahat Elwakeel, Rasha Elsayed Mohamed, Study of Pentraxin-3 as an Early Marker of Diabetic Nephropathy in Type 2 Diabetes Mellitus, International Journal of Diabetes Research, Vol. 7 No. 3, 2018, pp. 41-49. doi: 10.5923/j.diabetes.20180703.01.
Article Outline
1. Introduction
- Diabetes Mellitus (DM) is a real problem all over the world and Egypt [1], where the international Diabetes Federation listed Egypt among the world top 10 countries in the number of diabetics [2]. The prevalence of type 2 diabetes mellitus (T2DM) in Egyptian society is around 15.56% among adults, but in the future, it is expected to grow [3]. With population growth, aging, economic development, the increasing prevalence of obesity and physical inactivity, it is estimated that the total number of people with DM will more than double from 171 million in 2000 to 366 million in 2030, [4]. The systemic inflammatory reaction in DM has been an important cause of microvascular complications. [5] Diabetic nephropathy is the most common complication of diabetes and the leading cause of end-stage renal disease in developed countries [6, 7].Pentraxins (PTXs) are super family of soluble, multifunctional, pattern recognition proteins [8]. Pentraxins are structurally divided into two groups: short pentraxins and long pentraxins. CRP and amyloid P-component are classic short pentraxins produced by the liver after stimulation by interleukin 6 (IL-6) whereas PTX3 is a prototype for a long form, produced locally at the inflammatory sites by several cell types, primarily mononuclear phagocytes, fibroblasts, dendritic cells, smooth muscle cells and endothelial cells in response to inflammatory signals [9]. PTX3 is positively associated with development and progression of diabetic retinopathy [10]. These data suggest the role of PTX3 as a biomarker for vascular diseases. Several studies showed that type 2 DM was associated with the increases of acute phase proteins such as C-reactive protein (CRP), fibrinogen, plasminogen activator inhibitor, cytokines and chemokines [11-14].Aim of the study: To evaluate serum level of Pentraxin-3 in patients with type 2 diabetes mellitus and to assess its correlation with high sensitivity C reactive protein (hs-CRP) and urinary albumin exertion as predictor markers for early diabetic nephrology.
2. Patients and Methods
- Patients:This case control prospective study was conducted on 60 patients with type 2 diabetes (T2D).Patients were recruited from Internal medicine and endocrinology departments in Al-Zahraa University Hospital during the period from March 2017 to January 2018. From all subjects participating in the study an oral consent was taken. Also approval of the ethical committee of faculty of medicine, AL-Azhar University was obtained.Inclusion criteriaAdult patients with type 2 diabetes mellitus with age more than 18 years, not hypertension, or ischemic heart disease, and with body mass index of less than 30 kg/m2, with normal renal function.Exclusion criteria:Patients with acute or chronic known infection, cardiovascular (CVD), hypertension, autoimmune disease, malignancy, pregnancy lactation, using corticosteroid and patients with renal impairment were excluded from the study.MethodsAll subjects in this study were subjected to the following:Full medical history and complete physical examination.Anthropometric measures: body mass index (BMI): weight divided by the square of the height (Kg/m2) and waist circumference.Laboratory assessment as: complete blood count (CBC), liver function tests, kidney function tests, fasting blood sugar, post prandial blood sugar, glycated haemoglobin (HbA1c), lipid profile, measurement of serum pentraxin-3 and serum hs-CRP were done for all subjects and 24 urinary albumin excretion for patients only. All these laboratory assessment were done and repeated from March 2017 to January 2018.Sample collection and storage: Five ml of venous blood were withdrawn under complete aseptic technique from the fasting patients and control in a sterile vacutainer tube 1ml for complete blood picture were taken on EDTA solution, 3ml of the samples were taken in plain tube, left to clot, centrifuged at 2000×g for 10 minutes and sera were separated without delay. The routine investigations (liver function, kidney function and fasting blood sugar) were done on the same day. The rest of the serum sample were stored frozen at -20 C after careful labelling till the time of the assay of serum pentraxin-3 and hs-CRP.Complete blood picture (CBC) were done on sysmex KN21, Fasting and post prandial blood sugar, Liver function tests (Serum albumin, bilirubin, alanine transaminases (ALT) and aspartate transaminase (AST), Kidney function tests (serum urea and creatinine) and lipid profile, All of them were done on cobas 311 clinical chemistry auto-analyzer from ROCH diagnostic company. Serum pentraxin-3 (PTX3) was measured by using quantitative sandwich enzyme immunoassay (EIA) method and the kit was supplied from bioassay technology (Reader Aз 1851 & Washer 909) from das. (Italy), Cat. No E1938Hu. [15]. Hs-CRP was determined by a solid phase immunosorbentassay (ELISA) and the kit was supplied by DRG International Inc (841 Mountain Avenue, Springfield, New Jersey, USA) [16].Albumin concentration in urine was measured using a Minineph micro albumin kit based on nephlometry method on Minineph-nephelometer (AD200) (The Binding Site, Birmingham, UK) [17].Estimated GFR (eGFR) was calculated by using Cockcroft–Gault formula:
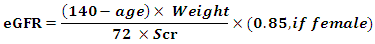 Where, GFR = glomerular filtration rate [ml/min]; SCr = serum creatinine concentration; CCr = creatinine clearance.SCr is in milligrams per deciliter, age is in years, and weight is in kilograms [18].Statistical Analysis:We calculate sample size according to Raosoft and all statistical calculation were done using Statistical Package for Social Science (IBM SPSS) version 20. Quantitative data were presented as mean, standard deviations and ranges when parametric. The comparison between two groups regarding quantitative data with parametric distribution was done by using Independent t-test. Spearman correlation coefficients were used to assess the correlation between two quantitative parameters in the same group. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant as the following: P > 0.05: Non-significant. P < 0.05: Significant. P < 0.01: Highly significant.
Where, GFR = glomerular filtration rate [ml/min]; SCr = serum creatinine concentration; CCr = creatinine clearance.SCr is in milligrams per deciliter, age is in years, and weight is in kilograms [18].Statistical Analysis:We calculate sample size according to Raosoft and all statistical calculation were done using Statistical Package for Social Science (IBM SPSS) version 20. Quantitative data were presented as mean, standard deviations and ranges when parametric. The comparison between two groups regarding quantitative data with parametric distribution was done by using Independent t-test. Spearman correlation coefficients were used to assess the correlation between two quantitative parameters in the same group. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant as the following: P > 0.05: Non-significant. P < 0.05: Significant. P < 0.01: Highly significant.3. Results
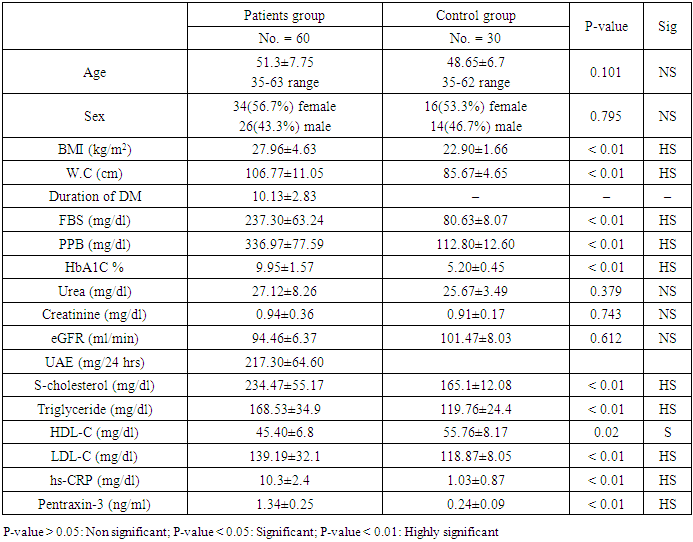
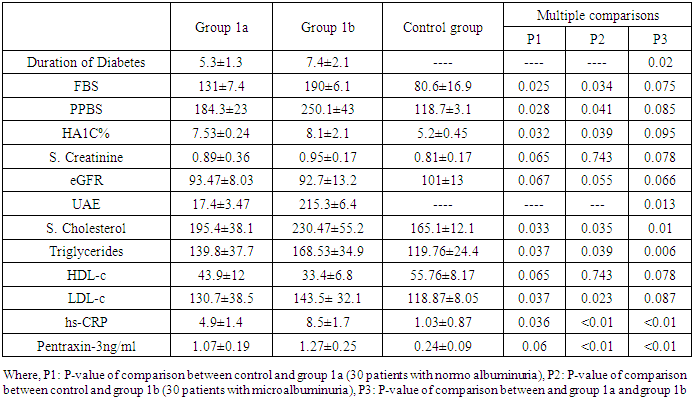
- Demographic Data of Studied Groups ShowedThis case control prospective study was conducted on 60 patients with type 2 diabetes (T2D) they were 26 males and 34 females. Their ages ranged between (35-63years) with mean ± SD (51.03±7.75). Duration of diabetes ranged between (3-8years), with mean SD (6.85±1.34) years (group 1) and also age matched 30 apparently healthy subjects were taken as a control. They were 14 males and 16 females. Their ages ranged between (35-62) years with mean ± SD (48.6±6.7), (group 11).According to the urinary albumin excretion our patients (group 1) were divided into two sub-groups:Group 1a: included 30 diabetic patients with normo albuminuria (UAE less than 30 mg/24 h) they were 18 females (60%) and 12 males (40%) their ages ranged between (35-59) years with mean ± SD of (49±5.62) years.Group 1b: included 30 diabetic patients with micro albuminuria (UAE more than 30 mg/24h and less than 300 mg/24h) they were 16 females (53.3) and 14 males (46.7) their ages ranged between (38-63) years with mean ± SD of (51.1±6.32) years.There were highly significant increases in the mean ± SD of body mass index (BMI), waist circumference, fasting blood sugar, postprandial blood sugar, haemoglobin A1C in Group I as follows (27.96±4.63), (106.77±11.05), (237.30±63.24), (336.97±77.59), (9.95±1.57), when compared to Group II with the mean ± SD of (22.90±1.66), (85.67±4.65), (80.63±8.07) (112.80±12.60) (5.20±0.45) respectively with p<0.01. while, There was no significant difference between group I and Group II as regard of blood urea, serum creatinine, estimated glomerular filtration rate (eGFR), with (p>0.05) table 1.
|
|
|
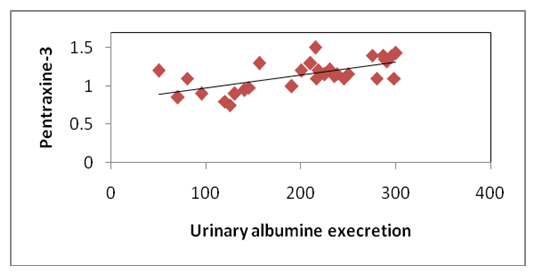 | Figure 1. Correlation of pentraxine-3 and urinary albumin excretion in group 1b |
|
4. Discussion
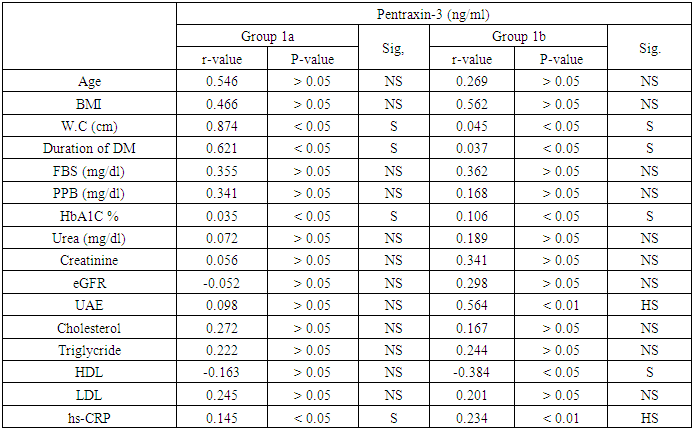
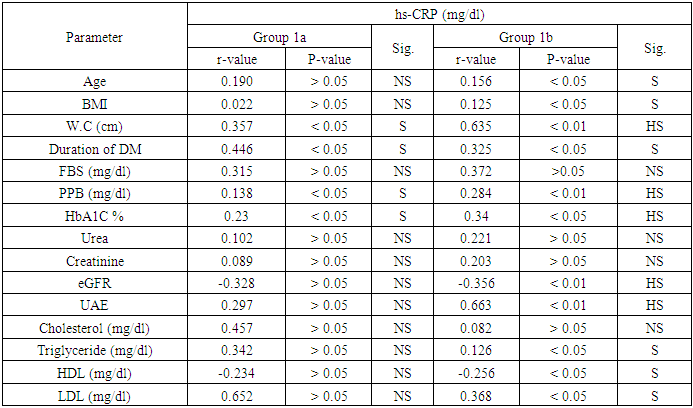
- In our study, there were statistically significant increases in serum levels of pentraxin-3 and hs-CRP in all patients with type 2 diabetes (group 1) compared to controls (group 11). These elevations are more significant in diabetic patients with micro albuminuria (group 1b) than in diabetic patients with normal albuminuria (group 1a).Moreover, there was significant positive correlation between serum pentraxin-3 and each of hs-CRP, HbA1c in both diabetic groups (group 1a and group 1b) and a positive correlation between serum pentraxin-3 and each of waist circumference, duration of diabetes and urinary albumin excretion in group 1b was noted.Wang and his colleagues (2016) [19] reported that serum pentraxin-3 concentration was significantly higher in patients with T2DM in comparison to control in their study which was done on 160 patients with T2DM and 54 healthy subjects. Also they reported that serum pentraxin-3 was positively correlated with urinary albumin excretion and this is in agreement with our results.IN agreement with our results a Chinese study which was done by DUAN et, al. (2013) [20] they found that serum pentraxin-3 was significantly higher in diabetic patients with micro albuminuria when compared to normo albuminuric diabetic patients. Also serum pentraxin-3 and urinary albumin excretion was positively correlated. Also an Egyptian study was done by Amr et. al., (2013) [21] who measured serum pentraxin-3 to detect endothelial dysfunction in diabetic patients compared to Von Willebrand factor (vWF) activity and reported that serum level of pentraxin-3 was significantly higher in micro albuminuric group and significant positive correlation between serum pentraxin-3 and each of HbA1c and duration of diabetes.Our study in agreement with [22] who, showed that serum pentraxin-3 was significantly higher in diabetic patients compared to controls but their study was on patients diabetic polyneuropathy.Another study agree with our results [23] who found significantly elevation of pentraxin-3 in patients with type diabetes and proteinuria with normal renal function when compared with healthy volunteers. Also Yilmaz et. al., 2009 [25] agree with our results.As regard correlation of serum pentraxin-3 with studied parameters we found that there was significant positive correlation between serum pentraxin-3 and each of duration of diabetes, waist circumference, HbA1c and hs-CRP in both diabetic groups. And non-significant positive correlation between pentraxin-3 and serum cholesterol, triglyceride and low density lipoprotein was noted.Our results agree with [21] they reported that there was significant positive correlation between pentraxin-3 and duration of diabetes, HbA1c and fasting blood sugar. But disagree with our results as regard correlation of pentraxin-3 and each of serum cholesterol, triglyceride where they reported significant positive correlation. As regard urinary albumin excretion our results revealed significant positive correlation between it and serum pentraxin-3 which is agree with [19, 20].Our results agree with [25] who found that pTX3 significaly higher in patients with diabetes and also significantly correlated with HbA1c and urinary albumin excretion.In contrary to our results [26] who reported that serum pentraxin-3 was lower in diabetic patients with diabetic nephropathy in comparison to patients without diabetic nephropathy.In our study we found that fasting blood sugar, post prandial blood sugar, HbA1c levels were higher than the levels set by American Diabetes Association. This is more increase in group 1b and this is poorly controlled glycaemic status might provide explanation for endothelial dysfunction in early diabetic nephropathy.AS regard our results about serum level of hs-CRP we found significantly elevation of it in all patients with type 2 diabetes in comparison to controls.In agreement with our study [27, 28], they showed that serum hs-CRP was significantly elevated in type 2 diabetic patients compared to healthy controls.Many previous studies are in consistent with our results [24, 29, 30] and also [31] demonstrated a liner increasing trend in serum hs-CRP for normal glucose tolerance through impaired fasting glucose level, impaired glucose tolerance and newly diagnosed diabetes mellitus. Our results showed significant elevation of serum hs-CRP in (micro albuminic group (group 1b) in comparison to normoalbuminuric group (group 1a) which is in agreement with [32] who reported for the first time that serum CRP levels are associated with development of diabetic nephropathy and urinary album level is increased by 1.02mg/24h for each increase in CRP of 1mg/L over 10 years of follow up, [33] who showed that serum hs-CRP was significantly higher in diabetic patients with microalbuminuria.Extended previous studies are in agreement with our results [34, 35]. Kanwar, [36], showed that hs-CRP is the major acute phase protein and an established nonspecific marker of inflammation. A number of epidemiological studies have showed that Hs CRP is an important risk factor for atherosclerosis and coronary heart disease. Pasceri and his colleagues, [37] reported that hs-CRP directly increased vascular cell adhesion molecules and intercellular adhesion molecule in human endothelial cells demonstrating the direct pro inflammatory effect of hs-CRP.Consistent with our results [38-41] they reported that hs-CRP was significantly elevated in patients with type 2 diabetes and more increased in patients with micro albuminuria.In our study there was significant positive correlation between serum hs-CRP and HbA1c in diabetic patients which is agree with [28] in their study who measured serum GGT activity and hs-CRP to evaluate oxidative stress, inflammation and glycemic control in type 2 diabetes mellitus Indian patients who found significant linear relation between hs-CRP and HbA1c and also [42] reported similar results.IN agreement with our [38] who concluded that serum hs-CRP is positively correlated with fasting blood sugar and HbA1c and this is more significant in micro albuminuric patients.Also Sangappa et., al. [27] agree with our study where they reported positive correlation between HbA1c and hs-CRP but it not statistically significant.IN consistent with our study [43] reported that high levels of hs-CRP were correlated with high levels of HbA1c and fasting blood sugar.As regard correlation of hs-CRP and urinary albumin excretion we found significant positive correlation between them which is in agreement with [34, 44] who showed that there was positive correlation between CRP level and micro albuminuria in diabetic patients and these results suggested that low grade inflammation which is reflected by high serum hs-CRP may play a role in induction of micro albuminuria, which can be considered as a risk factor for cardiovascular disease [45].Also Yunqian et, al [38], and mohd et., al. [33] agree with our results and reported that high CRP level associated with the development of diabetic nephropathy.Consistent with our results [39] reported that elevated levels of hs-CRP are associated with the development of diabetic nephropathy but not associated with the progression of nephropathy.
5. Conclusions
- The study concluded that serum pentraxin-3 (PTX3) and hs-CRP are significantly elevated in patients with type 2 diabetes and these elevations were more significant in patients with micro albuminuria and there was significant positive correlation between pentraxin-3 and hs-CRP as well as HbA1c, fasting blood and urinary albumin excretion in all patients with type 2 diabetes. The association between PTX3, hs-CRP and urinary albumin excretion suggest that PTX3 had a role as inflammatory marker for early detection of diabetic nephropathy.
6. Recommendations
- Measurement of serum PTX3 and hs-CRP can be used as screening method for early detection of diabetic nephropathy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML