-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2018; 7(2): 31-35
doi:10.5923/j.diabetes.20180702.02

Effect of DiaBliss Herbal Sugar (DHS) in Patients with Type-II Diabetes Mellitus
Arun Chockaligam 1, Dattatreya Rao 2, Sagar Sarikonda 3, Kodandarami Reddy 4, Damodar Reddy 3, N. Chidambaram 5, N. Saravanan 6, C. S. Janaki 7, Rajalakshmi 8
1Professor of Epidemiology, Medicine & Global Health, University Toronto, Toronto, Canada
2S. V. Ayurveda College, Tirupati, Andhra Pradesh, India
3Sugen Life Sciences Pvt Ltd, Tirupati, Andhra Pradesh, India
4Department of Anthropology, S. V. University, Tirupati, Andhra Pradesh, India
5Rajamuthaiah Medical College, Annamalai University, Chidambaram, Tamil Nadu, India
6Lecturer in Biochemistry, Rani Meyammai College of Nursing, Annamalai University, Chidambaram, Tamil Nadu, India
7Department of Anatomy, Asan Memorial Dental College & Hospital, Chengalpet, Tamil Nadu, India
8Chief Technology Officer, DiaBliss Consumer Products Pvt Ltd, Thoraipakkam, Chennai, Tamil Nadu, India
Correspondence to: C. S. Janaki , Department of Anatomy, Asan Memorial Dental College & Hospital, Chengalpet, Tamil Nadu, India.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Diabetes mellitus is a major public health problem both in developed and developing countries. Treatments with life style management options are limited; continued efforts are being made to identify nutrients which offer beneficial effect in the management of diabetes. Objective: To study the effect of long-term administration of DiaBliss Herbal Sugar (DHS) in diabetic patients. Methods: The study was conducted in two phases. In the first phase glycemic index of DHS was determined in 16 healthy volunteers against standard glucose in a non-blind, repeated measure, cross-over design. In the second phase, a prospective observational clinical trial was conducted in 100 adult diabetic patients of both genders and DHS was administered (25 g/day) for 90 days. A baseline fasting blood sample was obtained on day zero and thereafter, three more blood samples were taken every 30 days. The samples were analyzed for the fasting blood glucose, HbA1c, lipid profile and safety parameters. Results: The glycemic index (GI) value of DHS was found to be 46.5, and may be considered as a low glycemic nutrient/food. Long-term administration of DHS does not increase blood glucose and the lipid profile was not altered. We have noted a decrease in HbA1c and serum alkaline phosphatase levels, but these initial findings need further investigations. Conclusion: DHS appears to be safe and does not increase the blood glucose and lipid levels in type-II diabetes patients. Adverse effects were not observed and there seems to be a decrease in HbA1c levels.
Keywords: Diabliss Herbal Sugar, Diabetes, Herbal Solution, Low Glycemic Index
Cite this paper: Arun Chockaligam , Dattatreya Rao , Sagar Sarikonda , Kodandarami Reddy , Damodar Reddy , N. Chidambaram , N. Saravanan , C. S. Janaki , Rajalakshmi , Effect of DiaBliss Herbal Sugar (DHS) in Patients with Type-II Diabetes Mellitus, International Journal of Diabetes Research, Vol. 7 No. 2, 2018, pp. 31-35. doi: 10.5923/j.diabetes.20180702.02.
Article Outline
1. Introduction
- Diabetes Mellitus is the most common chronic disease affecting millions of people worldwide. Altered life style and diet are the driving forces for the increased prevalence rate of diabetes [1, 2]. Due to its serious health complications, the management of diabetes warrants additional financial burden to people. Globally, it was estimated that diabetes accounted for 12% of health expenditures in 2010 at least $ 376 billion- a figure expected to reach $ 490 billion in 2030 [3]. As the daily average consumption of sugar has been significantly increased during the last two decades especially in developing countries like India, the diabetic epidemic shows an alarming trend. In spite of the availability of many effective medicines to treat diabetes, the chronic nature of the disease and maintaining strict diet and exercise remain the challenges in achieving better health in diabetic patients. For centuries, several herbs are being routinely used as substitutes to sugar in Indian subcontinent. Based on the lead by local and traditional health practitioners, tribal and Indian System of Medicine, a herbal sugar was prepared with the ingredients that exhibit anti-diabetic properties which include aqueous extracts of turmeric (rhizome), ginger (rhizome), fenugreek (seeds), black pepper (fruit), pomegranate (fruit seeds), cinnamon (bark), and gooseberry (fruit). Previous preclinical and clinical studies demonstrated that these extracts showed potent anti-diabetic, anti-inflammatory, hypolipedimic and anti-cancer activities with no evidence of side effects. Based on the safety nature of the above seven extracts, we developed a novel herbal sugar fortified with aqueous extracts of the seven medicinal plants. The present study hypothesized that long-term administration of DiaBliss Herbal Sugar (DHS) is unlikely to increase the blood glucose levels in diabetic patients. Patients consumed the DHS as nutritional supplement for the current study and it could be alternative for sugar in future prospective to main good health.
2. Materials and Methods
- Preparation of DHSThe herbal solution is an aqueous extract of seven herbs viz., Turmeric (rhizome), Ginger (rhizome), Fenugreek (seeds), Black pepper (fruit), Pomegranate (fruit seeds), Cinnamon (bark), Gooseberry (fruit). Each of the herbs is individually extracted using a unique process. Individual aqueous extracts are then filtered and blended to yield a colorless and odorless solution. DHS is a novel product manufactured by using a proprietary process wherein regular cane sugar is blended for 30 min with the herbal solution (40 ml/ kg of sugar). After the blending, the wet sugar was unloaded into a container and left undisturbed overnight. Subsequently, the sugar was dried at 60°C to obtain the final consumable product in a crystalline form which is indistinguishable from regular sugar. No change in physical (color), or chemical property was observed for the resulting modified sugar and after this preparation is called Diabliss Herbal Sugar (DHS).Subjects Group-ISixteen healthy adult subjects were recruited for the study. Subjects were moderately active, non-smoking and non-alcoholics. Exclusion criteria were as follows: age, 35-55 years; BMI >25 kg/m2; fasting blood glucose value >120 mg% because fasting blood glucose in the range of 110 to 125mg/dl indicated impaired fasting glucose or pre diabetes. Subjects Group -IIA total of 100 diabetic patients (Type-2) who were in the age range of 30 to 55 years with fasting blood glucose levels in between 150-200 mg/dL who were on standard medication (i.e. Glimepiride) were considered. The exclusion criteria were: patients with diabetes associated retinopathy, nephropathy and cardiovascular disease; lactating women, patients with recent stroke and heart attack, abnormal blood urea and creatinine. Following a glucose tolerance test (GTT) which is more substantial indicator of diabetes than finger prick testing, written informed consent was obtained from each patient after the process was explained in their local language.
3. Study Design
- Subjects were recruited from the outpatient clinic at Raja Muthiah Medical Collage Hospital as well as special diabetes camps around Chidambaram, Tamil Nadu. Patients were given DHS at 25g/day packed in small sachets for 90 days and the patients were asked to consume the same amount at different times of the day (two or three times / day). At the beginning and at the end of the study the glycosylated hemoglobin, lipid profile, kidney and liver function tests were performed. The fasting blood glucose levels were measured at the beginning of the study, 7, 30, 60 and 90 days after commencement of DHS intake to determine the glycemic index by using the International standard method for measuring glycemic index (GI) of DHS. On the day prior to the test, subjects were asked to restrict their activities and not to eat or drink after 21.00 hours the night before the test, although water was allowed. Test food: 50 grams of DHS. The standard food consisted of 50 g of glucose. Glucose tolerance test (GTT) was performed. After an overnight fasting (12h) the blood sample was collected and the subjects were asked to consume the glucose or DHS in a crossover model on a different day. The gap between the two GTT tests was a minimal of three days after completing the first test with glucose. A fasting intravenous blood sample was taken at Zero min and glucose or DHS was consumed immediately after this. Further blood samples were taken at 30, 60, 90 and 120 days for the estimation of blood glucose by glucose oxidase method followed by HbA1c, Insulin were measured [5]. Ethics committee approval was obtained before the initiation of the trial. Ethical approval for the study group I was obtained from the Snehal Hospital Ethics Committee, Mumbai. The recruitment of patients from different locations of India will give us better results in understanding the dietary patterns influence in regulation of blood glucose levels along with DHS supplementation. Ethical approval for the study group II was obtained from Raja Muthiah Medical College Institutional Ethics Committee in Chidambaram. Subjects were given full details of the study protocol and the opportunity to ask questions. All subjects gave written informed consent prior to participation in the study. The protocol used was in line with the procedures recommended by the Food and Agriculture Organization/World Health Organization. Statistical analysis was carried out using SPSS 10.0 and p values were set at 0.05 as significant. Repeated measures of ANOVA and students‘t’ was applied to observe the changes in end parameters.
4. Results
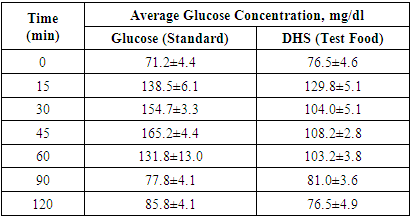
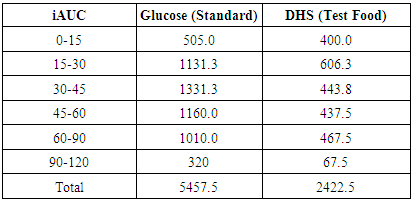
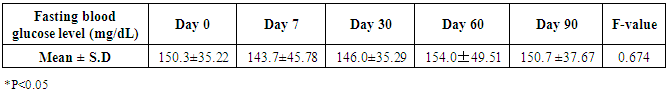
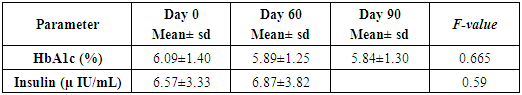
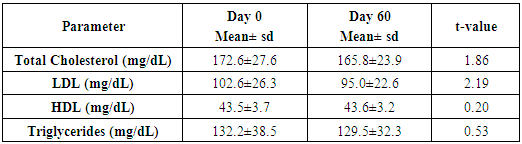
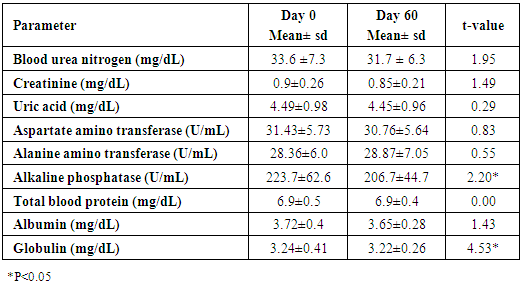
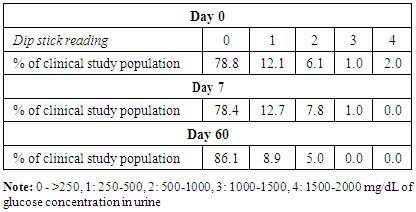
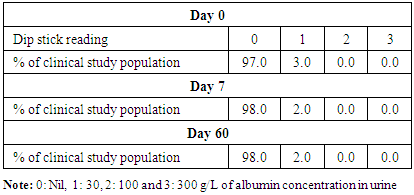
- Administration of DHS exhibited low GI value compared to standard glucose in healthy subjects. The changes in blood glucose levels were lower upon the administration of DHS when compared to standard glucose (Figure 1). GI values are often grouped into categories as producing either a low, medium or high glycemic response. The cut-off GI values are as follows: low <55, medium 56-69, high >70. The GI of DHS was found to be 46.5 and thus the DHS can be grouped as a low glycemic food [Table 1 & 2]. Fasting blood glucose levels were measured in diabetic patients on day 0, 7, 30, 60 and 90 days after DHS consumption. Consumption of DHS for 90 days do not show any significant change in the mean fasting blood glucose levels [Table-3]. The mean level of HbA1c in 100 subjects was 6.09. The results suggest that there was a decreasing trend in the HbA1c at the end of the study period compared to day zero, but it was not statistically significant. No statistically significant differences in the mean levels of insulin (Mention was not made in methodology) were observed between day zero and at the end of the study period. These results indicate that consumption of DHS does not alter HbA1c and insulin [Table-4]. The mean levels of lipid parameters (total cholesterol, HDL-cholesterol, LDL-cholesterol and triglycerides) were almost similar between the two intervals (day ‘0’ and 60) indicating that long term administration of DHS for 60 days does not adversely influence the key lipid parameters [Table 5]. Daily consumption of DHS did not show any significant changes in serum blood urea nitrogen (BUN), creatinine, uric acid, aspartate amino transferase (AST), alanine amino transferase (ALT), alkaline phosphatase (ALP) and blood protein levels [Table 6]. Furthermore, urine glucose levels as measured by qualitative dip stick method showed a lowering trend whereas urine albumin levels remained unchanged [Table 7 & 8].
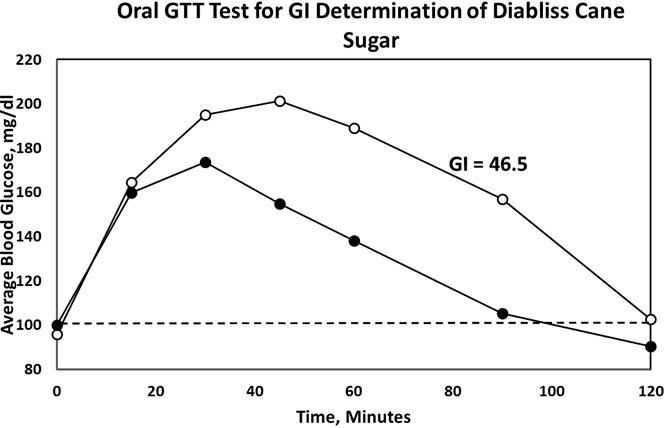 | Figure 1. Average blood- glucose levels at different time intervals after glucose or DHS Consumption |
|
|
|
|
|
|
|
|
5. Discussion
- Lowering postprandial blood glucose levels and glycemic load is one of the current methods in the management and prevention of obesity and diabetes [7, 8]. Pharmacological therapy is evidently effective, a holistic combination of lifestyle changes, functional foods/nutraceuticals along with drugs can be more effective in not only delaying the onset of diabetes but also in achieving better glycemic control in pre-diabetic and diabetic populations. Daily intake (25 g/day) of functional food-DHS (herbal sugar fortified with antidiabetic plant extracts) for 90 days maintains blood glucose levels in diabetic patients in the present study clearly indicating that consumption of DHS in place of conventional sugar may be beneficial thereby reducing the diabetic complications. It has been reported that intake of low GI diets is associated with glycemic decrease, and lower and more consistent post-prandial insulin release, avoiding the occurrence of hypoglycemia. Consumption of a low GI diet has been shown to be beneficial in reducing body weight, total body fat and visceral fat, levels of pro-inflammatory cytokine markers and the occurrence of dyslipidemia and hypertension [9, 10]. Consumption of functional foods that show anti-diabetic properties along with standard drugs to control blood sugar is likely to be more effective in glycemic control. Regulation of post-prandial blood sugar levels is the most important aspect in the management of diabetes [11, 12]. The health of diabetics and pre-diabetics, obesity, lipid oxidation and body composition, childhood management of obesity and reducing the risk of metabolic disease are associated with benefits of low glycemic index diet [13]. Many functional foods have been shown to be beneficial in diabetes. Many functional ingredients from both plants and animals have been described for the treatment of diabetes throughout the world [14, 15, 16]. Preparation of different food products using DHS may markedly reduce their GIs without modifying their ingredient profile and taste. Given the interest and potential benefits of low GI diets, further studies of DHS with a larger study population and in combination with other high GI foods are likely to reveal more beneficial effects of DHS. The use of DHS may potentially have positive health implications and further research is therefore warranted to confirm the long-term benefits of this novel herbal sugar.
6. Conclusions
- Long-term consumption of DHS maintains normal blood sugar levels and is safe in view of the unaltered lipid and other safety markers like liver and renal function tests thus indicating the safety of DHS. Decreasing trend of HbA1c levels shows that there is a beneficial effect on the average blood sugar levels although a similar study with a larger population for longer duration is required to confirm the efficacy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML