-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2018; 7(2): 23-30
doi:10.5923/j.diabetes.20180702.01

Metallothionein-1A Gene +1245 A/G Polymorphism (rs8052394) is not Associated with Impaired Glucose Regulation (IGR) and Type 2 Diabetes Mellitus (T2DM) in Bangladeshi Population
Marjia Rahman1, Sohel Ahmed1, Hussain Md. Shahjalal1, Manisha Kalita Das2, Zahid Hassan2, Liaquat Ali3
1Department of Biochemistry and Molecular Biology, Jahangirnagar University, Savar, Dhaka, Bangladesh
2Department of Physiology and Molecular Biology, Bangladesh University of Health Sciences, Darus Salam, Mirpur-1, Dhaka, Bangladesh
3Department of Biochemistry and Cell Biology, Bangladesh University of Health Sciences, Darus Salam, Mirpur-1, Dhaka, Bangladesh
Correspondence to: Sohel Ahmed, Department of Biochemistry and Molecular Biology, Jahangirnagar University, Savar, Dhaka, Bangladesh.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Metallothionein-1A (MT1A) is a cysteine-rich protein, which detoxifies toxic metals as an antioxidant. Polymorphisms in MT1A gene have been implicated to the pathogenesis of type-2 diabetes mellitus (T2DM), although some studies have shown ambiguous results in different population groups. In this study, we investigated the frequency distribution of MT1A gene +1245 A/G polymorphism (rs8052394) and its association with impaired glucose regulation (IGR) and T2DM in Bangladeshi population. 284 subjects aged 35-65 years were recruited, all of whom have undergone a two-sample oral glucose tolerance test (OGTT). Based on OGTT, 114 subjects were categorized as control, 42 as IGR and 128 as T2DM. The MT1A +1245 A/G polymorphism was examined by PCR-based RFLP assay. The heterozygous (AG) genotype frequencies were very low in control (13.2%), IGR (4.8%) and T2DM (14.8%) subjects. The frequencies of G allele were also very low in control (0.066), IGR (0.024) and T2DM (0.074) subjects. Statistical analyzes suggest that gene +1245 A/G genotype and allele frequencies did not show any significant associations with IGR and T2DM (p=0.229 and p=0.668, respectively). The fasting-glucose level, but not the glucose level 2h-after glucose load, fasting-insulin and insulin-glucose ratio, was significantly higher in heterozygous variant compared to that with wild type only in T2DM subjects (p=0.022). This study suggests that MT1A gene +1245 A/G polymorphism is less frequent and not significantly associated with IGR or T2DM in the Bangladeshi population studied.
Keywords: Impaired glucose regulation (IGR), Type 2 diabetes mellitus (T2DM), Metallothionein-1A (MT1A), Non-synonymous single nucleotide polymorphisms (nsSNP)
Cite this paper: Marjia Rahman, Sohel Ahmed, Hussain Md. Shahjalal, Manisha Kalita Das, Zahid Hassan, Liaquat Ali, Metallothionein-1A Gene +1245 A/G Polymorphism (rs8052394) is not Associated with Impaired Glucose Regulation (IGR) and Type 2 Diabetes Mellitus (T2DM) in Bangladeshi Population, International Journal of Diabetes Research, Vol. 7 No. 2, 2018, pp. 23-30. doi: 10.5923/j.diabetes.20180702.01.
Article Outline
1. Introduction
- Metallothioneins (MTs) are a class of metal-binding proteins characterized by high cysteine content [1]. Up to seven divalent metal ions, such as Cadmium, Zinc, Copper, can be bound to human MTs and this binding stabilizes the three-dimensional structure of MTs [2]. MTs can be activated by a variety of stimuli, including metal ions, cytokines and growth factors [3-5]. They are highly expressed when exposed to oxidative stress or toxic factors [6, 7]. Several reports suggest that MTs detoxify heavy metal cations, scavenge free radicals, and thus protect cells against cytotoxic [8, 9], radiation and DNA-damaging effects [10-13]. It has been shown that the loss of the protective effects of MTs leads to an escalation of pathogenic processes and carcinogenesis. However, despite their important actions in preventing oxidative damage, regulation of MT biosynthesis in diseased tissues is not well characterized [14].Different physiological and pathological conditions can exert oxidative stress-induced damages in human [15]. In type 2 diabetes mellitus (T2DM), oxidative stress markers are elevated leading to improper management of glycemia and relentless deterioration of cell function [16]. Mutations of MT genes resulting in MT proteins devoid of scavenger function of reactive oxygen species can lead to the predisposition and/or the pathogenesis of T2DM [17, 18]. Kang (1999) also reported that MT significantly protects heart from a variety of oxidative stimuli in MT-overexpressing transgenic mouse [19]. MTs were also implicated in the prevention of diabetic cardiotoxicity in mouse model [20-23]. Taken together; these results suggest that MTs would be a candidate for antioxidant therapy to prevent various pathological conditions including diabetes mellitus.MTs are genetically polymorphous protein families with four main isoforms expressed in humans. The isoforms are MT1, MT2, MT3, and MT4 [24]. Despite the physical-chemical similarity of the forms, their roles and occurrence in tissues vary significantly [25]. MT1 and MT2 are present almost in all types of soft tissues [26-28], MT3 is expressed mostly in brain tissue, but also in heart, kidneys and reproductive organs [29, 30] and the MT4 is detected in stratified squamous epithelial cells associated with oral epithelia, esophagus, upper stomach and neonatal skin [31]. MT1 and MT2 regulate copper and zinc homeostasis, detoxify heavy metals, play a role in immune function, and are involved in a variety of gastrointestinal tract functions. MT3 functions as a growth inhibitory factor in the brain. It plays a major role in the development, organization and programmed death of brain cells. MT4 helps regulate stomach acid pH, taste and texture discrimination of the tongue and help protect against sunburn and other skin traumas [32]. In humans, the MT genes are located on chromosome 16 in a cluster [33]. Although the MT1, MT3 and MT4 proteins are encoded by a single gene, the MT1 protein comprises many subtypes encoded by a set of 13 MT1 genes. The known active MT1 genes are MT-1A,-1B, -1E, -1F, -1G, -1H, -1M and -1X. The rest of the MT1 genes (MT1C,-1D,-1I,-1J and 1L) are pseudogenes that are not expressed in humans [33]. Three different single nucleotide polymorphisms (SNPs), rs11640851, rs8052394 and rs1610216, in MT genes were shown to be associated with diabetes and its complications [17, 34, 35]. The rs8052394 is a non-synonymous SNP of MT1A gene which is located 152 nucleotides from the first codon (ATG) (c.152A/G) and it causes a substitution of lysine by arginine at 51 position of the MT1A protein (Lys51Arg polymorphism). This polymorphism may bring some conformational change leading to decrease in stability of the protein, as predicted by MuPro software [36]. Mutant MT1A protein cannot perform its normal antioxidant function, so cells may become susceptible to oxidative damage which in turn leads cellular damage and pathological conditions. The nsSNP rs8052394 is common in Asian populations [36]. Yang et al (2008) showed significant associations of the frequency distributions of the G allele in MT1A gene SNP rs8052394 with the incidence of T2DM and decreased serum superoxide dismutase activity in Chinese people of Han descent [17]. In contrast, Ganasyam et al (2012) showed that there is no significant association of the MT1A gene rs8052394 polymorphism in Indian women of T2DM [37]. However, in Bulgarian cohort, Kozarova et al (2012) reported that the single nucleotide polymorphism +1245 A/G MT1A gene is related with coronary artery disease but not with diabetes. They also showed different distributions of +1245 A/G MT1A polymorphism among healthy controls and diabetic patients without cardiovascular disease and patients with coronary artery disease [38]. Thus, previous studies on the possible role of MT1A gene polymorphism in T2DM have shown ambiguous results [17, 37, 38].Like many other countries, T2DM is the most common form of diabetes in Bangladesh. Some of the established polymorphic genetic markers implicated in the pathogenesis of T2DM and its complications have been studied for the understanding of the pathogenetic mechanism of T2DM in Bangladeshi population. However, the association of MT1A genotype variation with the risk of T2DM in Bangladeshi population has not been established. In the present study, we investigated the frequency distributions of MT1A gene +1245 A/G polymorphism (rs8052394) and its association with the newly diagnosed IGR and T2DM subjects of Bangladeshi origin. The association of MT1A gene polymorphism with several serum measurements including fasting and 2h glucose, fasting insulin, and insulin/glucose ratio were also examined. Results of our study may help in further understanding of the pathogenesis of T2DM in Bangladeshi population.
2. Subjects and Methods
2.1. Subjects
- A total of 284 Bangladeshi subjects aged 35-65 years were purposively enrolled in the study irrespectively of their race, religion and socioeconomic status. Among them 114 (male: 59, female: 55) were healthy controls, 42 (male: 22, female: 20) were IGR and 128 (male: 70, female: 58) were T2DM subjects. Healthy subjects having no serious disease were recruited through personal communication from the community. Diagnosis of IGR and T2DM was made by oral glucose tolerance test (OGTT) according to WHO criteria [39]. IGR and T2DM patients under antihyperlipidemic medications were excluded in this study. Pregnant women, lactating mother and patients with serious comorbid diseases such as stroke, myocardial infarction, liver cirrhosis, major surgery, renal failure and/or infections were also excluded. Informed consent was obtained from all participants at the Out-Patient Department of the Ibrahim Memorial Diabetes Centre, Dhaka, Bangladesh. The study was approved by the Ethical Review Committee of the Diabetic Association of Bangladesh.
2.2. Anthropometric Measurements
- Height: Standing height was measured without shoes using appropriate scale (Detect-Medic, Detect scales INC, USA). Height was recorded to the nearest 5 mm. Weight: The balance was placed on a hard flat surface and checked for zero balance before measurement. The subjects were in the center of the platform wearing light cloths without shoes. Weight was recorded to the nearest 0.5 Kg. Body mass index (BMI): BMI (Kg/m2) of each subject was calculated using following formula:
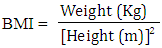
2.3. Collection of Blood Sample
- Overnight fasting (8-10 hours) blood was collected between 8.00-9.00 am. Venous blood (10 ml) was obtained by venipuncture following standard procedure. Subjects were then given glucose to drink (75 g in 300 ml of water). They were requested not to take any food and be rested for two hours. The second blood sample was then obtained. A portion of blood (5 ml) sample was taken into a tube containing EDTA (1 mg/ml), mixed thoroughly and preserved at -30°C for future DNA extraction and subsequent experimentation. Fasting and postprandial blood samples were taken into plain tube allowed to clot for 30 minutes and serum was separated by centrifugation for 10 min at 3000 rpm and preserved at -30°C for further biochemical analyses.
2.4. Biochemical Analyses
- Glucose was estimated by enzymatic colorimetric (GOD-PAP) method in Hitachi 704 Automatic Analyzer (Hitachi Ltd., Tokyo, Japan) using glucose estimation kit (Randox Laboratories Ltd., UK). The reagent kit contains peroxidase and glucose oxidase enzymes along with phenol and 4-aminophenazone. The serum sample or standard (5 μl) was mixed with 500 μl reagent and incubated at 37°C for 10 minutes. The enzyme glucose oxidase catalyzes oxidation of glucose into gluconic acid with the formation of hydrogen peroxide. The hydrogen peroxide then reacts with phenol and 4-aminophenazone under catalysis of peroxidase to form a red violet quinoneimine. Then, the absorbance for the sample and the standard against the reagent blank were measured at 500 nm within 60 minutes. Finally, serum glucose concentration of each individual was calculated based on the absorbance of the sample and standard. Insulin was measured by Enzyme Linked Immuno Sorbent A ssay (ELISA) using human insulin assay kit (Linco Research Inc., USA) following standard laboratory procedure. This assay was based on: i) concurrent capture of human insulin molecules from serum samples to the wells of a microtiter plate coated by a pre-titered amount of monoclonal anti-human insulin antibodies and the binding of a second biotinylated monoclonal mouse anti-antibody to the captured insulin, ii) washing of unbound materials, iii) binding of horse radish peroxidase (HRP) enzyme to the immobilized biotinylated antibodies, iv) washing of free enzyme conjugates, and v) quantification of immobilized antibody-enzyme conjugates by monitoring horse radish peroxidase (HRP) activities in the presence of the substrate 3,3′,5,5′-tetramethylbenzidine (TMB). The enzyme activity is measured spectrophotometrically at 450 nm. The absorbance of the formed product is directly proportional to the amount of captured human insulin in unknown serum sample. The serum insulin concentration of each individual was then determined by interpolating a reference curve generated in the same assay with reference standards of known concentrations of human insulin.
2.5. DNA Extraction
- Extraction of DNA was performed using FavorPrep™ Blood DNA Extraction Kit (FAVORGEN®, Taiwan). The kit uses the principal of silica gel DNA isolation from whole blood adapted in spin column.
2.6. Polymerase Chain Reaction (PCR) of MT1A Gene
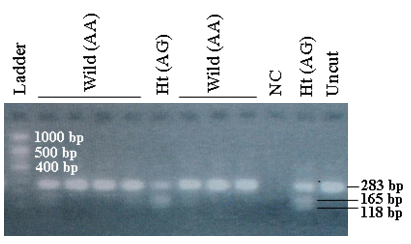
- PCR was carried out using the following primer set according to the protocol described previously [17].Forward: 5′-ACTAAGTGTCCTCTGGGGCTG- 3′Reverse: 5′-AATGGGTCACGGTTGTATGG- 3′ PCR product size for the primer set is 283 bp. PCR was carried out using HotStar Taq polymerase. Conditions for the amplification included initial step of denaturation at 95°C for 15 minutes followed by 35 cycles of denaturation at 94°C for 45 seconds, annealing at 55°C for 30 seconds and elongation at 72°C for 30 seconds and finally a step of final elongation at 72°C for 10 minutes. PCR assays were performed in a DNA Thermal cycler (Biometra, Germany). A negative control (reagent blank), which contained all components of the reaction mixture except the sample DNA, was included in every PCR run.
2.7. RFLP Analysis of MT1A Gene Candidate Markers
- MT1A gene candidate polymorphic markers +1245 A/G were analyzed using a site-specific restriction enzyme, PstI. The enzyme digestion was performed following standard protocol and the candidate markers were detected by restriction fragment length polymorphism (RFLP) assay (Figure 1).
2.8. Statistical Analyses
- Statistical analyses were performed using Statistical Package for Social Science (SPSS) software for Windows version 17 (SPSS Inc., Chicago, Illinois, USA). The statistical differences between groups were assessed by Unpaired Student’s ‘t’ test. Chi-square test was performed to assess the association between groups. A two-tailed p value of < 0.05 was considered statistically significant.
3. Results
3.1. MT1A +1245 A/G Genotype and Allele Frequencies of the Study Subjects
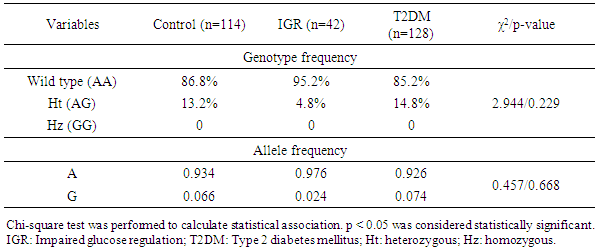
- Genotype frequencies of the MT1A gene +1245 A/G polymorphic markers were 86.8% and 13.2% for wild type and heterozygous (Ht) variant, respectively, in the control group. In the IGR group, the frequencies were 95.2% and 4.8%, respectively, and in the T2DM group, the frequencies were 85.2% and 14.8%, respectively. The genotype frequency distributions in both IGR and T2DM groups did not show any significant associations (χ2=2.944; p=0.229) (Table 1). The frequencies of A and G alleles in MT1A gene +1245 A/G polymorphism were 0.934 and 0.066, respectively, in the control group. In the IGR group, the frequencies were 0.976 and 0.024, and in the T2DM group, the frequencies were 0.926 and 0.074, respectively. The allele frequency distributions in both IGR and T2DM groups also did not show any significant associations (χ2=0.457; p=0.668) (Table 1).
|
3.2. MT1A Gene +1245 A/G Genotype Frequencies in the Male and Female Subjects
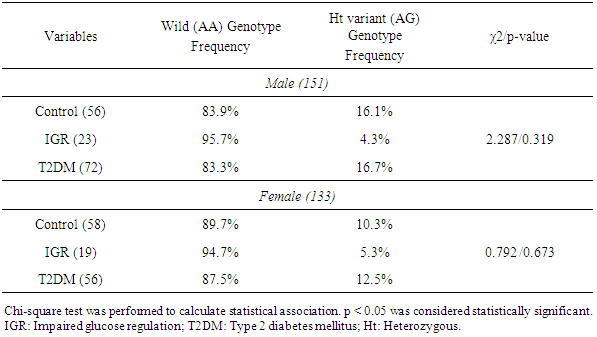
- MT1A gene +1245 A/G genotype frequencies were also analyzed separately in male and female control, IGR, and T2DM subjects. The distributions did not show any significant associations either in male (p=0.319) or female (p=0.673) IGR and T2DM subjects (Table 2).
|
3.3. Fasting Glucose, 2h-AG Glucose, Fasting Insulin Levels and Insulin-Glucose Ratio in the Wild and Variant Genotypes of the Control, IGR and T2DM Subjects
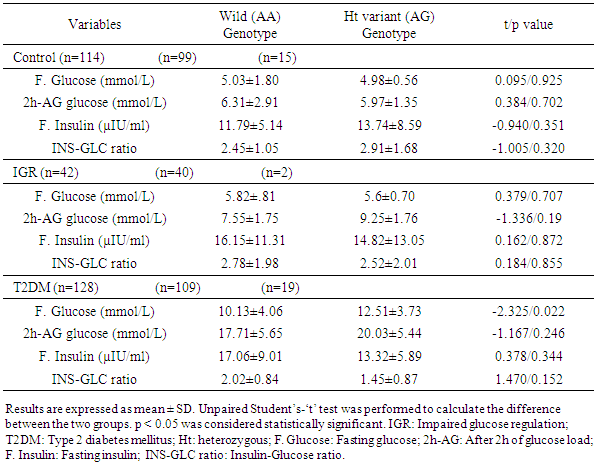
- Glucose levels (both fasting and 2h after glucose load), fasting insulin levels and insulin-glucose ratio were compared in MT1A +1245 A/G wild and variant genotype of the control, IGR and T2DM subjects (Table 3). Serum glucose levels, fasting insulin levels and insulin-glucose ratio did not show any significant differences between the wild and variant genotype in the control and IGR subjects. In contrast, fasting glucose level was significantly different between the wild and variant genotype in the T2DM subjects (p=0.022), with variant genotype had significantly higher level (12.51±3.73 mmol/L) compared to those with wild genotype (10.13±4.06 mmol/L). However, serum glucose level 2h after glucose load, fasting insulin level and insulin-glucose ratio did not show any significant differences between the wild and variant genotype in the T2DM subjects.
|
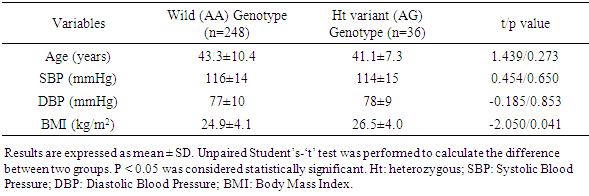
3.4. Age, Blood Pressure, and BMI of the Study Subjects with MT1A +1245 A/G Wild and Variant Genotype
- Age and both systolic (SBP) and diastolic (DBP) blood pressures of the subjects with MT1A +1245 A/G wild and heterozygous variant genotype did not show any significant differences (p=0.273 for age, p=0.650 for SBP, and p=0.853 for DBP). In contrast, body mass index (BMI) was significantly higher in the subjects with variant genotype compared to the subjects with wild genotype (p=0.041) (Table 4).
|
4. Discussion
- Diabetes mellitus is a chronic non-communicable disease having serious health, economic and social consequences [40]. Type 2 diabetes mellitus (T2DM) is the most prevalent form of diabetes worldwide. The underlying mechanism of the pathogenesis of T2DM remains unsettled. Many investigators believe that insulin resistance precedes beta-cell dysfunction which is the late phenomenon due to exhaustion after years of compensatory hyper-secretion [41]. Others claim that impaired beta-cell function is the primary underlying cause of T2DM resulting from genetic defect in the beta-cell secretory machinery [42]. Oxidative stress plays an important role in the pathogenesis of T2DM and its complications [15]. Pancreatic beta-cells are particularly sensitive to reactive oxygen species because they are low in antioxidant enzymes such as catalase, glutathione peroxidase, and superoxide dismutase. Therefore, the ability of oxidative stress to damage mitochondria and markedly blunt insulin secretion causing T2DM is not surprising [16, 43]. Metallothioneins (MTs) are metal binding stress-response antioxidant proteins. They play important antioxidative role in the defense against excessive essential metals and this protective function is related to the ability of MTs to scavenge free radicals [19, 44]. Several reports suggested the associations of MT gene polymorphisms with T2DM and coronary artery diseases [18, 38], while others showed no significant association with T2DM [37]. In the present study, we investigated whether the MT1A gene +1245 A/G polymorphism (rs8052394) has been playing any role in the susceptibility to IGR and T2DM, for the first time, in a considerable number of Bangladeshi subjects. The mean age of T2DM subjects (43.9±9.1 years) found in the study was comparable to the age of control (39.8±10.5 years) (p=0.101) (data not shown). This preludes the recruitment bias of case and possibility of incidental differences regarding biochemical and anthropometrical variables. It is noteworthy that mean age of IGR subjects (47.1±9.1 years) was significantly higher compared to the control (p=0.001). Obviously, it may be assumed whether this higher age of IGR results the true scenario of the Bangladeshi population. Data are, however, lacking about the conclusive comment on this issue.In the present study, we observed that the genotype frequencies of MT1A gene +1245 A/G in the control group were 86.8% (wild type, AA) and 13.2% (heterozygous, AG). In the IGR group, the frequencies were 95.2% (AA) and 4.8% (AG), whereas in the T2DM group, the frequencies were 85.2% (AA) and 14.8% (AG), respectively. These results indicate that heterozygous (Ht) variant in T2DM is similar to that of control subjects, but surprisingly very low in IGR. However, the genotype frequency distributions in both IGR and T2DM subjects do not any show significant associations (χ2=2.944; p=0.229). There is a similarity in the frequency of A allele in the control (0.934), IGR (0.976) and T2DM (0.926) subjects. But, A allele frequency is much more higher than the frequency of G alllele in all three groups. Statistical analyses show that the allele frequency distributions of the candidate marker do not show any significant associations in both IGR and T2DM subjects. Collectively, our results suggest that MT1A gene A/G polymorphism (rs8052394) may not be associated with the risk of either IGR or T2DM in Bangladeshi people. Our results are in good agreement with Ganasyam et al (2012) [37]. They showed that there is no significant association of the MT1A gene rs8052394 polymorphism in Indian women of T2DM. In contrast, Yang et al (2008) reported significant associations of the frequency distributions of the G allele in MT1A gene SNP rs8052394 with the incidence of T2DM in Chinese people of Han descent [17]. However, in Bulgarian cohort, it has been reported that the single nucleotide polymorphism +1245 A/G MT1A gene is related with coronary artery disease but not with diabetes [38]. In our study no individual was found with homozygous GG (Hz) variant, and therefore it may be assumed that Hz variant may not be frequent in Bangladeshi population. The absence of homozygous variant in Bangladeshi population is consistent with that of other country population. Yang et al (2008) in their study did not find any individual with homozygous (GG) variant, but there were 32% and 27% subjects with AG variant between T2DM patients and controls [17], which were found lower in the present study (13.2%, 4.8% and 14.8% in control, IGR and T2DM, respectively). Kozarova et al (2012) in their study also demonstrated the absence of homozygous (GG) variant in their control [38]. They also reported that among diabetic patients without cardiovascular disease 4% were homozygous (GG) variant but at the same time 32.7% were with heterozygous (AG) variant. Considering our results, we conclude that MT1A gene A/G polymorphism may be less frequent in Bangladeshi population. We also investigated the genotype frequencies of MT1A gene +1245 A/G variants separately in male and female control, IGR, and T2DM subjects. However, results suggest that there were no significant associations of variant genotypes with IGR and T2DM in both male (p=0.319) and female (p=0.673) subjects.Fasting serum glucose levels have significant difference between wild (AA) and variant (AG) genotype in T2DM (p=0.022), but not in IGR and healthy controls. Glucose levels 2h after glucose load, fasting insulin and insulin-glucose ratio did not show any significant differences between the wild and variant genotype in T2DM, as well as, in IGR and controls. However, when insulin-glucose ratio was considered, the compromised nature of insulin secretion in the wild and variant genotype was exposed. The ratio was lower in the variant genotype compared to the wild type in IGR and T2DM subjects. This suggests that even if absolute insulin level is high but the secretory capacity has already been compromised in IGR and T2DM subjects with variant genotype.In the present study, subjects with heterozygous variant genotype were found to have significantly higher BMI compared to those with wild genotype (p=0.041). But, considering age and blood pressure (both SBP and DBP), there were no significant differences between wild and variant genotype (p=0.273 for age, p=0.650 for SBP and p=0.853 for DBP). Thus BMI, but not the blood pressures, may have the association with MT1A gene A/G polymorphism in Bangladeshi people. However, further study with larger population size needs to conclude this issue.
5. Conclusions
- In summary, non-synonymous +1245 A/G polymorphism (rs8052394) in MT1A gene may be less frequent and not significantly associated with IGR or T2DM in Bangladeshi population. Therefore, at present whether this could be used as another predictive indicator for T2DM in Bangladeshi population remains uncertain. However, the results of our study should be considered exploratory and further studies on MT1A gene polymorphisms in a larger number of populations may provide its usefulness as a potential genomic indicator to predict the risk of T2DM and its complications.
ACKNOWLEDGEMENTS
- We gratefully acknowledge the technical and financial support provided by the Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine and Metabolic Disorders (BIRDEM) and Diabetic Association of Bangladesh (DAB) to conduct this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML