-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2018; 7(1): 1-11
doi:10.5923/j.diabetes.20180701.01

Opuntia stricta Cladode Extract Reduces Blood Glucose Levels in Alloxan-induced Diabetic Mice
Aubrey Chichonyi Kalungia1, Mary Mataka1, Patrick Kaonga2, Angela Gono Bwalya1, Lavina Prashar3, Derick Munkombwe1
1Department of Pharmacy, University of Zambia, Lusaka, Zambia
2Department of Internal Medicine, University of Zambia, Lusaka, Zambia
3Department of Physiological Sciences, University of Zambia, Lusaka, Zambia
Correspondence to: Aubrey Chichonyi Kalungia, Department of Pharmacy, University of Zambia, Lusaka, Zambia.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Opuntia stricta (commonly called prickly pear cactus) is a natural plant that grows in some parts of Zambia where its fruits and cladodes are commonly consumed for nutritional and medicinal purposes, including glycaemic control among some patients with diabetes mellitus (DM). There is insufficient evidence whether Opuntia stricta indigenously growing in Zambia possess antidiabetic effects. Aim: To assess in vivo antidiabetic effects of the aqueous extract of Opuntia stricta cladodes in alloxan-induced diabetic mice. Methods: A laboratory-based experimental study was conducted involving 20 adult Swiss albino mice (Mus musculus) weighing 18-30 g. DM was induced using a single intraperitoneal dose of alloxan monohydrate 90 mg/kg. Opuntia stricta aqueous extract was administered orally and blood glucose levels (in mmol/L) monitored daily for 10 days. Results: Alloxan induced a 4- to 5-fold sustained increase in blood glucose levels at 72 hours after administration in mice. Within a 10-day experimental period, Opuntia stricta cladode aqueous extract (1 mg/kg) significantly reduced blood glucose levels in vivo (from 16.6 ± 1.4 mmol/L, 95% CI: 14.9-18.3 at baseline to 7.5 ± 1.0 mmol/L, 95% CI: 6.2-8.9 at endpoint, p < 0.001, n = 5). Similarly, at a dose of 2 mg/kg, the extract significantly reduced blood glucose levels (from 18.7 ± 4.6 mmol/L, 95% CI: 13.0-24.4 at baseline to 6.9 ± 1.7 mmol/L, 95% CI: 4.7-9.0 at endpoint, p = 0.001, n = 5). Opuntia stricta cladode aqueous extract attained a greater reduction in blood glucose levels compared to Glibenclamide 0.25 mg/kg. Opuntia stricta cladode aqueous extract demonstrated a presence of alkaloids, flavonoids, saponins, sterols, carbohydrates, phenols and tannins. Conclusion: Opuntia stricta cladode from Zambia demonstrates antidiabetic effects to reduce blood glucose levels in vivo.
Keywords: Opuntia, Prickly pear cactus, Cladode, Alloxan, Antidiabetic, Phytochemicals, Zambia
Cite this paper: Aubrey Chichonyi Kalungia, Mary Mataka, Patrick Kaonga, Angela Gono Bwalya, Lavina Prashar, Derick Munkombwe, Opuntia stricta Cladode Extract Reduces Blood Glucose Levels in Alloxan-induced Diabetic Mice, International Journal of Diabetes Research, Vol. 7 No. 1, 2018, pp. 1-11. doi: 10.5923/j.diabetes.20180701.01.
Article Outline
1. Introduction
- Diabetes mellitus (DM) is a chronic metabolic disease characterized by persistent hyperglycaemia that occurs either when the pancreas does not produce insulin (type 1) or when the body cannot effectively utilize insulin (type 2) [1]. In DM type 2, acquired signaling defects and insulin resistance develops due to alterations in the partitioning of lipids between the adipocyte and muscle or liver leading to the intracellular accumulation of triglycerides and intracellular fatty acid metabolites (fatty acyl CoA’s, diacylglycerol, and ceramides, among others) in the insulin-responsive tissues [2, 3]. Chronic hyperglycaemia predisposes an individual to a variety of complications such as diabetic ketoacidosis, neuropathy, nephropathy, retinopathy and an increased risk of cardiovascular disease [4]. According to the World Health Organization, the majority of people around the world have developed type 2 DM largely as a result of modifiable risks such as obesity and sedentary lifestyle [1]. Global estimates indicate a rise in DM prevalence from 4.7% in 1980 to 8.5% in 2014 with over 420 million people reported with the disease [5]. In Zambia, a low- to middle-income country in Southern Africa, the prevalence of DM was estimated at 3.1% in 2014 and is poised to increase due to the growing burden of non-communicable diseases (NCDs) in the population [6]. Due to an insufficient national health expenditure for the management of chronic NCDs, including challenges with accessibility and affordability of medicines, the majority of the Zambian population cannot easily afford the cost of out-of-pocket payment for DM medications and hospital stays [7]. Moreover, with approximately 80% of the populations in developing countries relying on traditional herbal medicines for primary health care [8], it is not surprising that the unconventional use of a number of indigenous herbal plant remedies for management of NCDs in Zambia has been on the rise despite the limited scientific evidence of their efficacy and safety [9]. Opuntia (commonly called the prickly pear cactus), a member of the Cactaceae family, is a tropical cactus plant that grows in arid and semi-arid regions of the world. In Central and South America, the plant is indigenously used for medicinal purposes to control a variety of illnesses, including DM [10, 11]. Like in other countries in Sub-Sahara Africa [12], Opuntia species grow naturally in many parts of Zambia where its leaves and fruits are commonly consumed for possible nutritional and medicinal benefits, including glycaemic control among some diabetic patients. Current scientific evidence demonstrates that Opuntia cactus cladodes contain carbohydrates, vitamins, antioxidants and various flavonoids, particularly quercetin 3-methyl ether, a highly efficient radical scavenger [13, 14] and other phytochemical constituents that contribute to its potential hypoglycaemic and hypolipidaemic effects [15]. Opuntia extracts has been shown to significantly lower blood glucose levels [12, 16-19], triglycerides and total serum cholesterol levels [13-15] in animals. However, there is limited local evidence that has elucidated the phytochemical constituents and potential antidiabetic effects of Opuntia stricta variety indigenously growing in Zambia. We set out to determine the potential antidiabetic effect of Opuntia stricta cladode indigenously growing in Zambia in alloxan-induced diabetic mice and to screen the crude extract for presence of phytochemical constituents. Also to compare glycemic effect of the crude extract to Glibenclamide, a conventional antidiabetic drug used clinically for DM management.
2. Materials and Methods
2.1. Collection and Identification of Plant Material
- Plant specimens of Opuntia stricta cladodes (prickly pear cactus pads) were collected in March 2016 from Kabwata Township in Lusaka district, Zambia. The plants were growing at an altitude of approximately 1,283 m (4,290 ft) above sea level. The harvested specimens were botanically identified by plant taxonomists at the University of Zambia, Department of Biological Sciences. Laboratory and experimental procedures were then undertaken at the University of Zambia, Department of Physiological Sciences in Lusaka, Zambia.
2.2. Preparation of Crude Aqueous Extract
- Fresh cladodes of Opuntia stricta were first washed carefully with tap water to remove debris, then washed with sterile water, pruned and cut into small pieces. The samples were pulverised using a blender (5-minute rotations done three times) until consistent. Aqueous extraction method using molecular-grade sterile water as described elsewhere [20] was used. Approximately 100 g of the pulverised sample was placed in an extractor containing 20 ml of sterile water for 48 hours. The mixture was heated and brought to boil for approximately 20-30 minutes. The mixture was then allowed to cool and filtered through a Buchner’s funnel using suction filtration. The aqueous crude extract was then placed in an oven at 40°C and dispersed to form a thick gel. A percentage yield of 50% was attained. The extract was stored refrigerated at 6 – 8°C.
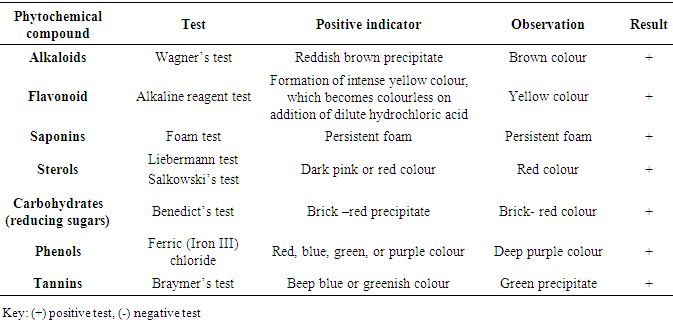
2.3. Phytochemical Qualitative Analysis
- The crude aqueous extract was assessed for the presence of phytochemicals using standard methods as previously described [21-24].
2.3.1. Test for Alkaloids
- Crude aqueous extract (2 ml) was measured into a test tube, and 2 ml of dilute hydrochloric acid (HCl) was added. The solution was shaken well and filtered. Wagner’s test: A few drops of Wagner’s reagent (a solution of iodine in potassium iodide) was added to the filtrate and observed for the formation of a reddish-brown precipitate which indicates the presence of alkaloids.
2.3.2. Test for Sterols
- Liebermann’s test: Two millilitres acetic acid and 2 ml chloroform were added to 2 ml of crude aqueous extract. The mixture was cooled and concentrated sulphuric acid (H2SO4) was added. A green colour was indicative of the presence of steroidal glycosides.Salkowski’s test: Two millilitres of crude aqueous extract was treated with 2 ml of concentrated H2SO4. Formation of a reddish-brown colour in the lower layer indicated the presence of sterols.
2.3.3. Test for Tannins
- Braymer’s test: Two millilitres plant extract was treated with 10% alcoholic ferric chloride solution. A deep blue or greenish colour showed the presence of tannins.
2.3.4. Test for Flavonoids
- Alkaline reagent test: Two millilitres of crude aqueous extract was treated with 2 ml of 2.0% sodium hydroxide (NaOH) solution. An intense yellow colour was produced, which became colourless on addition of 2 drops of dilute HCl. This indicated the presence of flavonoids.
2.3.5. Test for Saponins
- Froth test: To 2 ml of crude aqueous extract in a test tube, 2 ml sterile water was added, and the mixture was shaken vigorously. A positive test was confirmed by the formation of a persistent froth/form in the test tube, which indicated the presence of saponins.
2.3.6. Test for Reducing Sugars (Carbohydrates)
- Two millilitres of the crude aqueous extract was dissolved in 2 ml of sterile water and filtered. Benedict’s test: The extract filtrate was treated with equal volumes of Benedict’s reagent (sodium citrate, sodium bicarbonate and copper sulphate solution) in a test tube. The mixture was then boiled for 5-10 minutes in a water bath. Appearance of a brick-red precipitate indicated the presence of reducing sugars.
2.3.7. Test for Phenols
- Ferric chloride test: To 2 ml of crude aqueous extract, a few drops of dilute ferric (iron III) chloride solution was added. Formation of a reddish, green or purple coloured solution indicated the presence of phenols.
2.4. Test Animals
- Twenty (20) healthy adult Swiss albino mice (Mus musculus) weighing between 18-30 g were used. The animals were housed in polypropylene cages and maintained under standard conditions and room temperature (21-25°C). The animals were fed a standard mouse pellet diet and provided water ad libitum throughout the experimental period. All animals were allowed to acclimatize to laboratory conditions for 7 days before experimentation.
2.4.1. Induction of Persistent Hyperglycaemia (diabetes mellitus) in Mice
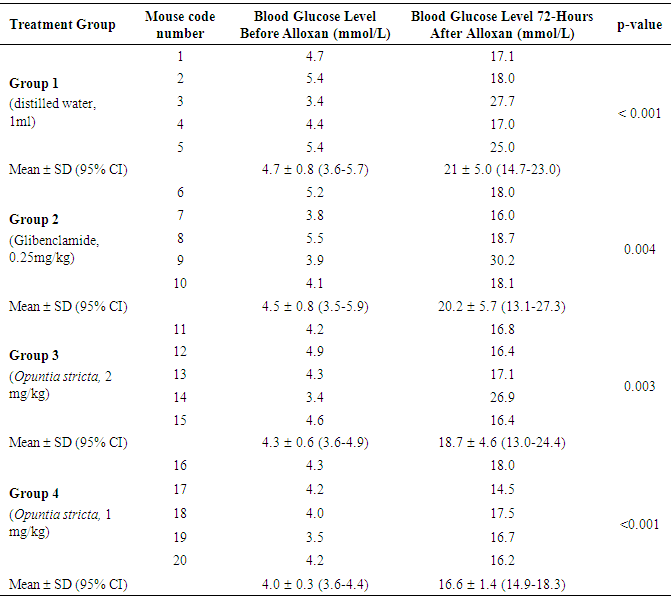
- The 20 mice were fasted overnight. A persistent hyperglycaemic state (DM) in the mice was induced using alloxan monohydrate (Sigma-Aldrich, St. Louise, USA) administered intraperitoneally at a single dose of 90 mg/kg body weight [20]. Alloxan monohydrate was freshly prepared as described elsewhere [25] and the solution kept on ice prior to use. The dose of alloxan used for induction of DM in mice was calculated using the following formula: dose volume = dose of alloxan / 1000 × body weight of mouse / concentration of alloxan.At 1 hour post-alloxan administration, the animals were continued on standard feed ad libitum and kept under observation and monitoring for 72 hours for development of hyperglycaemia. Blood glucose levels were measured (in mmol/L) from a drop of blood collected from a tail vein puncture [26]. A digital Accu-Check® glucometer (Roche Diagnostics, Mannheim, Germany) was used to measure blood glucose levels. Animals with baseline blood glucose level constantly above 9.0 mmol/L after 72 hours were considered to have developed persistent hyperglycaemia (DM) and were included in the experiment. After 72 hours, the diabetic mice were then randomly divided into four different experimental groups with each group containing at least 5 mice.
2.5. Experimental Design
- A laboratory-based, randomized control trial design using a randomized complete block method was used. A total of 20 alloxan-induced diabetic mice were randomly assigned to different experimental groups as follows: Group 1 (n = 5): Received 1 ml molecular grade sterile water orally once daily for 10 days (Control Group).Group 2 (n = 5): Treated with Glibenclamide 0.25 mg/kg body weight orally once daily for 10 days (Standard).Group 3 (n = 5): Treated with Opuntia stricta aqueous extract 2 mg/kg body weight orally once daily for 10 days. Group 4 (n = 5): Treated with Opuntia stricta aqueous extract 1 mg/kg body weight orally once daily for 10 days. Amounts of Opuntia aqueous extract administered were determined as previously described [19]. All animals were allowed free access to a standard mouse pellet diet and provided water ad libitum throughout the experimentation. The respective treatment was indicated in writing on the housing cage and animal welfare monitored daily in accordance with standard guidelines [27].
2.6. Data Collection
- Data for the phytochemical screening was collected by recording the respective test outcomes and observations (Table 1). In each animal, blood glucose level was measured before and after the respective treatment and recorded onto a data entry sheet daily for 10 days.
2.7. Data Analysis
- Experimental data that was normally distributed for each group were expressed as the mean ± SD. A paired t-test was used to compare mean blood glucose levels within groups before and 72 hours after alloxan administration, whereas an unpaired t-test and one-way ANOVA followed by Dunn’s post hoc test were used to compare means between groups. The phytochemical test result was descriptive and reported as present (+) or absent (-). Statistical analyses were conducted using GraphPad Prism version 5.01 software (GraphPad Software Inc. La Jolla, California, USA). For all statistical tests, a p-value less than 0.05 was considered statistically significant at 95% confidence interval.
2.8. Ethical Considerations
- Ethics approval of the study protocol was granted by the University of Zambia, Biomedical Research Ethics Committee (IRB00001131 of IORG0000774). Standard requirements for the conduct of experiments on whole animals, including practice of good animal welfare and husbandry, were strictly adhered to throughout the study in accordance with laboratory standard operating procedures set by the International Animal Care and Use Committee of Biotechnology Research Institute [27]. All animals used were humanely euthanized in a desiccator with chloroform and disposed after the conclusion of the study.
3. Results
- Presence of alkaloids, saponins, sterols, carbohydrates, phenols, tannins and flavonoids in the aqueous extract of Opuntia stricta cladodes were confirmed using various tests (Table 1). Flavonoids and other phytochemical constituents of Opuntia species have been shown in other studies to possess hypoglycemic and hypolipidemic effects.
|
|
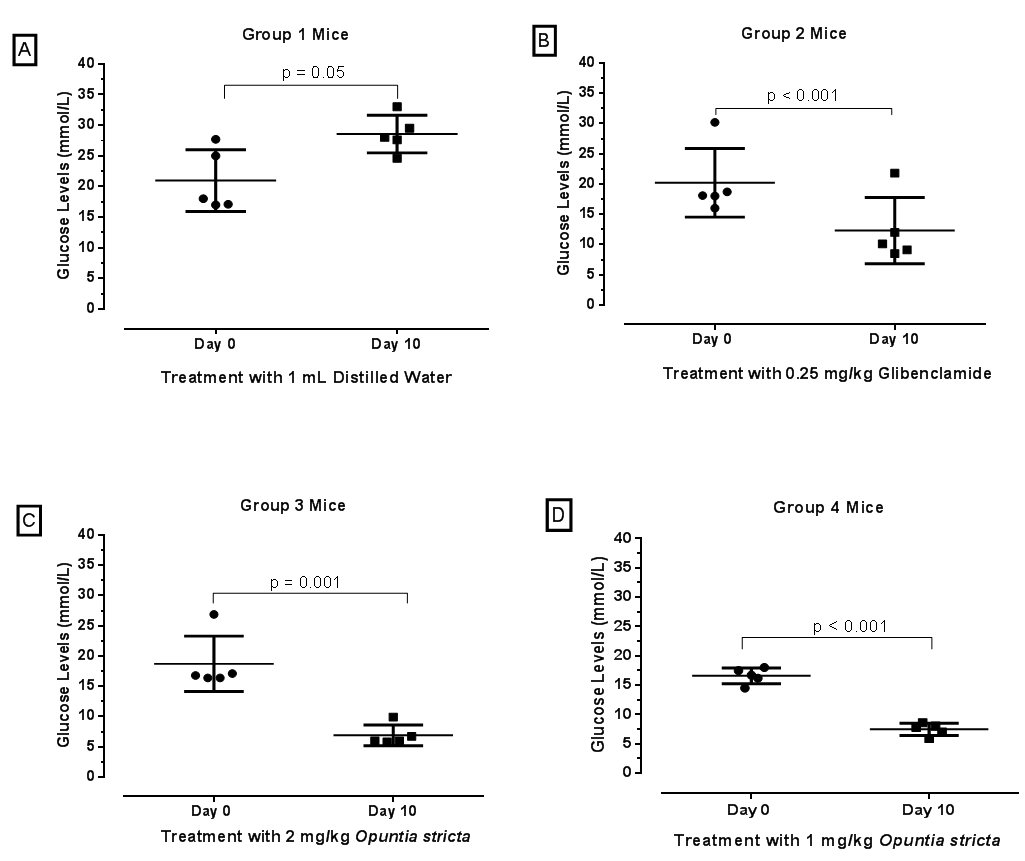
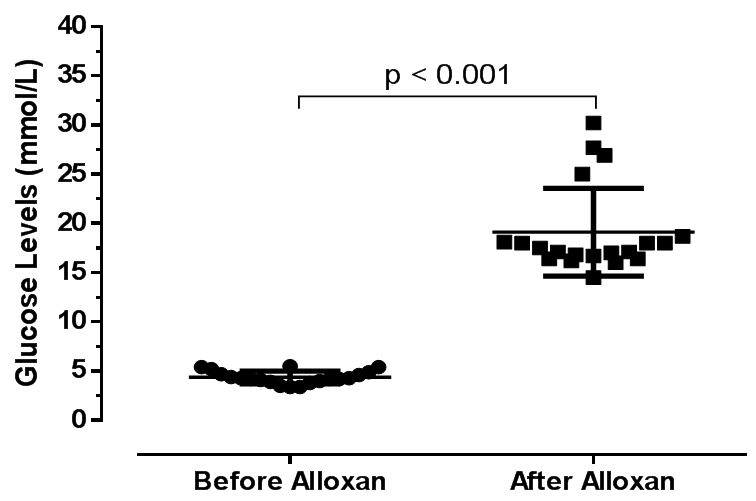 | Figure 1. Baseline blood glucose level in 20 mice before and 72 hours after administration of alloxan 90mg/kg |
|
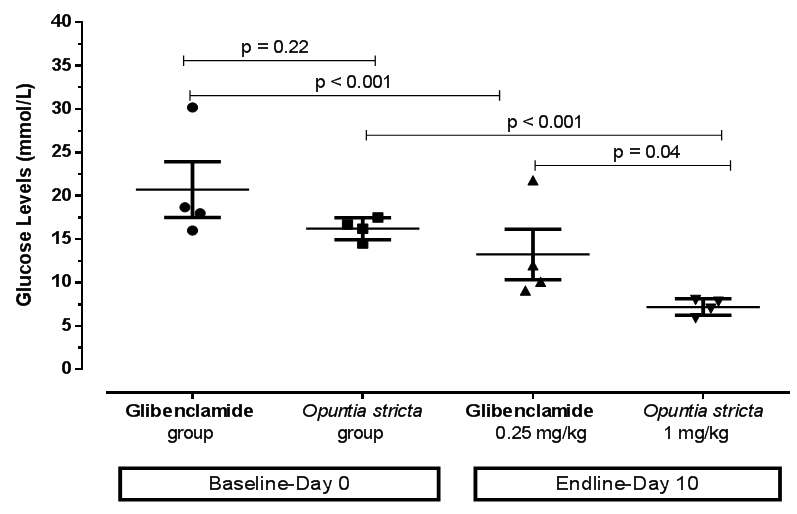 | Figure 3. Comparison of blood glucose levels of diabetic mice at baseline (day 0) and endpoint (day 10) after treatment with Glibenclamide and Opuntia stricta aqueous extract |
4. Discussion
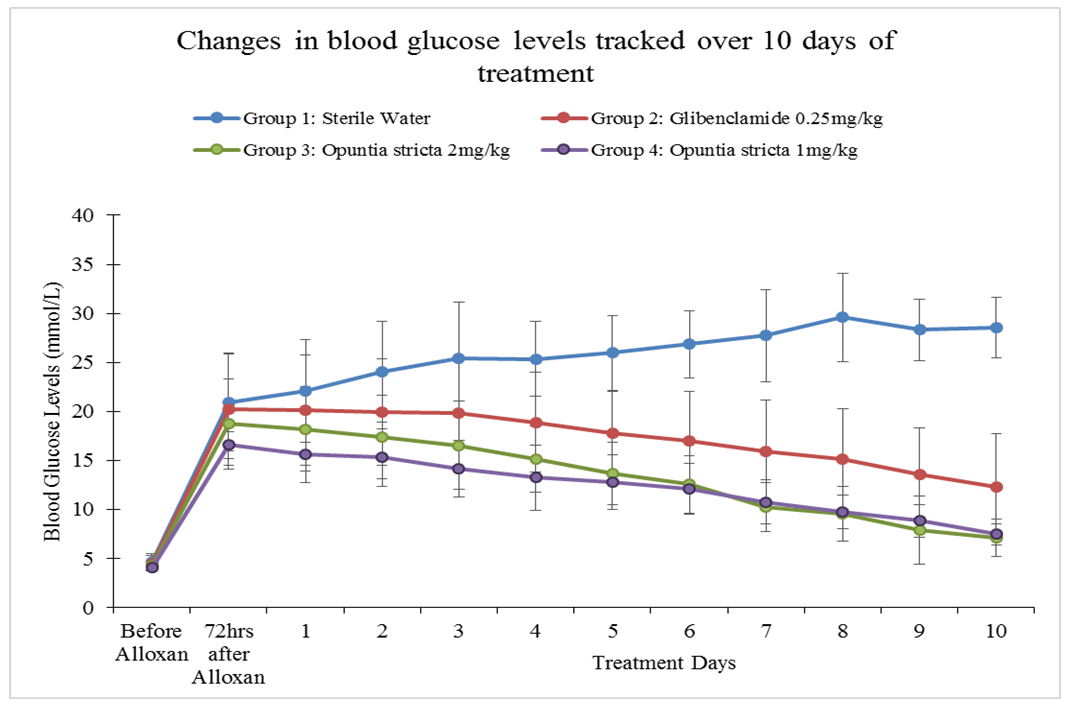
- A persistent state of hyperglycaemia is an important diagnostic indicator of DM. In this study, an aqueous extract of Opuntia stricta cladodes produced a significant reduction in blood glucose levels in alloxan-induced diabetic mice. Over a 10-day observation period, blood glucose levels in the diabetic mice treated with Opuntia stricta cladode extract tended to decline toward normal levels (Figure 4). Our findings showed that the test extract demonstrated a significant reduction in blood glucose levels, thereby controlling the DM state. This may suggest potential of Opuntia stricta cladodes to be studied further for possible use in DM management. We acknowledge that there are a number of species variation of Opuntia with varied number of phytochemical compounds that are determined by climatic and geographical factors [28]. The aqueous extract of Opuntia stricta cladodes harvested in Lusaka, Zambia were found to contain alkaloids, flavonoids, saponins, sterols, carbohydrates (polysaccharides), phenols and tannins (Table 1). According to Stintzing and Carle [14], cladode phytochemical composition tends to vary depending on the edaphic factors at the cultivation site, the season and the age of the plant. Whereas a plethora of evidence has demonstrated the presence of similar phytochemical constituents found in this study, comparative quantification of the actual phytochemical compounds found in different Opuntia species growing in Zambia and elsewhere remain to be isolated and elucidated. This is particularly useful especially since there are variations even among the more than 300 species of the plants found in different parts of the world [28]. It is envisaged that detailed qualitative elucidation of phytochemical compounds found in Opuntia stricta growing in Zambia is a potential scope of future studies. In this study, type 2 DM was pharmacologically induced through targeted destruction of the pancreas. Alloxan monohydrate at a 90 mg/kg dose sufficiently and significantly induced a persistent state of hyperglycaemia in the mice (Table 2). Being a pyrimidine derivative, alloxan is selectively taken up by insulin-producing pancreatic β-cells via the glucose transporter GLUT2 [29]. Because it is structurally similar to glucose, alloxan is easily and rapidly taken up by pancreatic cells. Within pancreatic cells, alloxan has a high affinity for sulphydryl groups and reacts with intracellular thiols, generating reactive oxygen species and free radicals that cause immediate cell damage. Additional evidence by Szkudelski [30] suggests that alloxan inhibits the glucokinase enzyme essential for glucose-induced insulin secretion. This, therefore, results in a state of insulin-dependent DM. As demonstrated in this study, our findings further support existing evidence that alloxan remains a powerful pharmacological agent for inducing DM in experimental rodents [31].The aqueous extract of Opuntia stricta cladodes produced a significant reduction in blood glucose levels in alloxan-induced diabetic mice (Figure 2) compared to no treatment (sterile water) and showed more reduction in blood glucose levels compared to standard treatment (Glibenclamide). Findings of this study demonstrate that the crude plant extract possesses pharmacological properties that produce antidiabetic effects in mice. Our findings also showed that even at a reduced dose (1 mg/kg), the test extract similarly produced significant reduction in blood glucose levels. Arguably, Opuntia stricta effects on blood glucose reduction were not dose-dependent. This could imply that the cladode extract was efficacious even at a reduced concentration. Whether this finding has implications on efficacy, safety and toxicity profile of the plant extract can be interesting to elucidate further. Although the plant has been widely consumed for medicinal benefits among populations in Zambia, this study was perhaps the first to find scientific evidence from an experimental study that the species of Opuntia stricta growing in Zambia showed antidiabetic effects. Our findings further support existing evidence [12, 18, 32] that similarly demonstrated the antidiabetic activity of other species of Opuntia (prickly pear cactus) extract in other settings. The relevance of these findings to stimulate further research cannot be understated because the evidence can promote detailed investigation into the chemical constituents of Opuntia and their putative mechanisms of action in DM. Though actual molecular mechanisms of antidiabetic effect in DM remain obscure, polysaccharide constituents of Opuntia sp. have been linked to beneficial effects in DM. For instance, Yang et al [33] suggested that the mechanism of polysaccharides found in Opuntia monacantha cladode hypoglycemic action might be similar to that of dimethylbiguanide – a conventional oral antidiabetic drug whose origins can be traced from the plant Galega officinalis [34, 35]. The hypoglycemic effect of dimethylbiguanide (Metformin) is mainly attributed to the inhibition of hepatic glucose output via the following proposed mechanisms: activation of APM-activated protein kinase (AMPK) through liver kinase B1 and decreased energy charge [36, 37]; the inhibition of glucagon-induced cAMP production by blocking adenylyl cyclase enzyme [38]; the increase of the AMP/ATP ratio by restricting NADH-coenzyme Q oxidoreductase (complex I) in the mitochondrial electron transport chain [39]; and more recently, the reduction of lactate and glycerol metabolism to glucose through a redox change by inhibiting mitochondrial glycerophosphate dehydrogenase [40]. Scholars also suggest that unabsorbed Metformin accumulates in the gut mucosa of the distal ileum delaying intestinal glucose absorption [41, 42]. Zhao et al [43] determined the most effective hypoglycemic component of polysaccharides from Opuntia dillenii Haw and preliminary screened the antidiabetic effects of O. dillenii polysaccharide (ODP)-Ia in mice with streptozotocin-induced DM. Three kinds of ODPs – ODP-Ia, ODP-Ib, and ODP-II′ – were isolated by using an ultrasonic extraction method and diethylaminoethyl (DEAE)-Sepharose fast-flow column chromatography. They postulated that ODP-Ia exerts its antihyperglycemic effect by protecting the liver from peroxidation damage and by maintaining tissue function, thereby improving the sensitivity and response of target cells in diabetic mice to insulin.Frati-Munari et al [18] studied different aspects of the hypoglycemic effect of Opuntia sp. and suggested that cactus pads reduce absorption of water-soluble dietary fibre content by interrupting absorption of glucose in the intestine. Similarly, Trejo-Gonzalez et al [19] assessed the blood glucose-reducing activity of purified extract from prickly pear cactus (Opuntia sp.) in streptozotocin-induced diabetic mice. They reported that although the mechanism of action was not known, the major substance that reduces blood glucose was presumed to be the dietary fibre in Opuntia extract. Hwang et al [16] classifies dietary fibre into water-soluble dietary fibre and non-water-soluble dietary fibre. Water-soluble dietary fibre is composed of mucus, gum, pectin, and hemicelluloses, while non-water-soluble dietary fibre is composed of cellulose, lignin, and a large hemicellulose fraction. The gel formed by water-soluble dietary fibre is known to prolong the passage of food through the intestine. Hwang et al [16] in their investigation of antidiabetic effect of fresh Nopal (Opuntia ficus-indica) in low-dose streptozotocin-induced diabetic rats fed on a high-fat diet revealed that both Nopal water extract (NPWE) and Nopal dry power (NPDP) showed inhibitory activity on 𝛼- glucosidase enzyme and prevent radical increase of blood glucose levels as decomposition into monosaccharide by 𝛼-glucosidase in the small intestine. Their study also verified the function of Opuntia aqueous extract as a hypoglycemic agent by confirming its inhibitory activity on 𝛼- glucosidase enzyme in-vitro. The forgoing evidence seem to agree with earlier evidence by Ou et al [44] that postulated three pathways by which bioactive constituents of Opuntia sp. produce antidiabetic effects; first by increasing the viscosity of the small intestinal content and retarding the diffusion of glucose. Secondly, by adsorbing glucose and preventing its diffusion and, finally, by inhibiting the activity of 𝛼-glucosidase enzyme and postponing the release of glucose from starch.Collaborative scientific evidence is required to support complementary use of Opuntia plant extracts in potentially reducing dosage requirements for conventional antidiabetic drugs such as sulphonylureas, biguanides and insulin via a considerable synergistic effect. For instance, Trejo-Gonzales et al [19] found that the combination of insulin and a purified extract of Opuntia fuliginosa Griffiths (1 mg/kg oral dose) reduced blood glucose and glycated haemoglobin levels to normal in rats compared to high quantities of parenteral insulin required for an equivalent hypoglycaemic effect. Other effects of Opuntia species that have been elucidated elsewhere include anticancer [45], antiviral [46], anti-inflammatory [47], and hypolipidaemic effects [14]. As suggested by Hegwood [48], scientific evidence on the nutritional and medicinal properties of Opuntia species may contribute to its increased use as a complementary and alternative natural product in the future. Despite its actual medicinal constituents remaining speculative, the plant may potentially provide a good, cheap source of alternative and complementary medicine for various ailments, especially in low- and middle-income countries where the cost of conventional medicines is high for the average patient. We only measured random blood glucose levels and not glycated haemoglobin which could have been more informative. Despite this limitation, blood glucose measurement remains a reliable evidence-based method [12, 25] of monitoring DM state. Although normally healthy mice were not used in this study, probably the findings on the primary outcome of interest investigated would have not differed significantly even if they were included. Moreover, the goal was to use a disease model that closely depicts the realities encountered in actual patient care. Since the principle aim of this study was to establish cause and effect, only one outcome (blood glucose level) was determined. However, other parameters such as changes in body weight and organ function (e.g., liver, kidney, and heart functions) were not measured. Detailed qualitative analysis of phytochemical constituents was beyond the scope of this study. In addition to developing alternative and complementary medicines for DM, we recommend that future studies to improve pharmacological management of DM can further explore the putative role of immune-modulation in preserving pancreatic beta cell function in type 1 DM and facilitating insulin secretion in type 2 DM. A growing body of evidence [49] demonstrates proof of concept.
5. Conclusions
- Opuntia stricta cladode aqueous extract reduced blood glucose levels in alloxan-induced diabetic mice in vivo. Opuntia stricta aqueous extract administered orally produced much greater reduction in blood glucose levels than Glibenclamide – a conventional oral antidiabetic drug used for DM management. Opuntia stricta cladodes from plants indigenously growing in Zambia demonstrates presence of carbohydrates (polysaccharides), alkaloids, flavonoids, saponins, sterols, phenols and tannins. Further elucidation of putative pharmacological mechanisms of action in DM control in vitro is required.
ACKNOWLEDGEMENTS
- Authors acknowledge the technical assistance rendered by technical staff at the University of Zambia from the Departments of Pharmacy and Physiological Sciences, respectively. We thank American Journal Experts (AJE) for English language editing.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML