-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2017; 6(4): 91-98
doi:10.5923/j.diabetes.20170604.03

Kisspeptin and Vaspin: Indicators of Insulin Sensitivity Improvement before Weight Loss Following Sleeve Gastrectomy in Experimental Type 2 Diabetes Mellitus
Mohamad Yosof Rezk , Hany Ahmed Elkatawy
Physiology Department Faculty of Medicine Zagazig University, Egypt
Correspondence to: Mohamad Yosof Rezk , Physiology Department Faculty of Medicine Zagazig University, Egypt.
| Email: |  |
Copyright © 2017 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Aim: detect changes in serum kisspeptin level and possible associated mechanisms in diabetic rats following sleeve gastrectomy (SG) specially the relationship between body wight (BW) and serum kisspeptin (Kiss). Animals and Methods: 40 rats were divided into 4 groups; Control (C), Diabetic (D), Diabetic Sham Operated (DSO) and Diabetic with Sleeve Gastrectomy (DSG) Group. Body weight (BW), Food Intake (FI) and serum Kisspeptin (Kiss) and Vaspin (V) levels were analyzed. Also, some serum glucostatic parameters including serum Glucose, Insulin and Homeostasis model assessment of insulin resistance (HOMA-IR) were measured. Results: BW increased significantly in D group in relation to C group (p=0.006). Insignificant changes in BW are found in DSG after 2 weeks in comparison with D group (p=0.8) however, significant reductions in body weight were found in DSG group after 4 and 10 weeks in comparison with D group (p=0.04 and 0.008 respectively). Kiss decreased significantly in D group in comparison with C group (p=0.04), however insignificant changes were found in DSO group at 2 w, 4 w and 10 weeks in comparison with D group (p= 0.5). Significant increases were found in Kiss in SG group after 2w, 4 w and 10 weeks in comparison with D group (p=0.0001). Significant increases were found in kiss DSG group after 2w, 4 w and 10 weeks in comparison with C group (p=0.03, 0.003, 0.0006 respectively). Vaspin (V) was decreased significantly in D group in relation to C group (p=0.0001), however insignificant changes in V were found in DSO group at 2 w, 4 w and 10 weeks in comparison with D group (p= 0.9). Significant increases in V were found in DSG group after 2w, 4 w and 10 weeks in comparison with D group (p= 0.0003, 0.0001). In Conclusion: Significant increases were found in Kisspeptin and Vaspin serum levels in DSG after 2w, 4 w and 10 weeks in comparison with C and D groups (p≤0.05). A moderate negative correlation (r= - 0.688) was found between serum Kiss and BW in all groups which means incomplete associaten and there is a tendency for Kiss to increase without corresponding reduction in body wight. Significant increases were found in Kiss in DSG groups after 2w, 4 w and 10 weeks in comparison with the D group (p=0.0001) without corresponding or associated significant reduction in BW at 2 weeks in comparison with D group (p=0.8). The significant increase in Kiss at week 2 with insignificant BW reduction indicates early changes in serum kisspeptin after sleeve gastrectomy preceding weight changes.
Keywords: Kisspeptin, Sleeve gastrectomy, Type 2 diabetes mellitus, Vaspin, Body weight
Cite this paper: Mohamad Yosof Rezk , Hany Ahmed Elkatawy , Kisspeptin and Vaspin: Indicators of Insulin Sensitivity Improvement before Weight Loss Following Sleeve Gastrectomy in Experimental Type 2 Diabetes Mellitus, International Journal of Diabetes Research, Vol. 6 No. 4, 2017, pp. 91-98. doi: 10.5923/j.diabetes.20170604.03.
Article Outline
1. Introduction
- Kisspeptins (Kiss) has been detected in the nervous system as well as peripheral tissues like placenta, testes and pancreas [1]. The Kiss-1 gene encodes 54, 14, 13 or 10 amino-acid peptides known as kisspeptins [2]. Actions of kisspeptins are conducted through G protein-coupled receptor 54 (GPR54) [3]. Kiss-1/GPR54 system has been included in tumor progression and metastasis and potent antimetastasis actions of Kiss-1 peptide have been found in thyroid and breast carcinoma [4]. Expression of Kiss-1/GPR54 system has been found in brain areas as hypothalamus, spinal cord, pituitary and human plasma [5] which strongly proves additional physiological functions of this peptide. It was found that there is a role for Kiss-1/GPR54 system in the neuroendocrine control of gonadotropin secretion, brain sex differentiation, puberty onset and fertility [6]. Hauge-Evans et al. (2006) have demonstrated the presence of kisspeptin and GPR54 mRNAs in both pancreatic B and A cells. Kisspeptin-54 has been shown to stimulate the late phase of glucose-induced insulin secretion in mouse and human islets [7]. A number of metabolic modulators have been found as regulators of kisspeptin like leptin, ghrelin, pro-opiomelanocortin (POMC) and neuropeptide Y (NPY) [8]. De Bond JA and Smith JT. (2014) reported that kisspeptin neurons can directly excite anorexigenic POMC neurons and indirectly inhibit orexigenic NPY neurons. They suggested that kisspeptin may have a direct role in regulating energy balance. They concluded that kisspeptin signaling may also be a direct regulator of metabolism. Expression of Kiss-1 in the hypothalamus is sensitive to nutritional status and it might contribute to the suppression of reproductive function in such conditions as negative energy balance periods [9]. Sagheb et al., (2017) reported that Kisspeptin (Kiss1) and its G protein-coupled receptor (GPR-54) (Kiss1r) is an essential component of controlling ghrelin expression in the hypothalamus and pancreatic beta cells express Kiss-1 and kissR. They suggested that ghrelin may have a similar role in the transcription of kiss1-KissR signaling in the pancreas too [10]. Type 2 diabetes mellitus (T2DM) is a prevalent disease that endangers human health, and searching methods to control the disease in a long-term and efficient manner is a worldwide problem [11]. Bariatric surgery can not only effectively reduce body weight but also relieve insulin resistance rapidly and permanently, a finding that has been confirmed in clinical studies [12]. Furthermore, bariatric surgery has been considered in the guidelines for treatment of T2DM [13, 14]. Sleeve gastrectomy (SG), the most widely used bariatric surgery [15], can significantly alleviate T2DM [16]. Vaspin is highly expressed in visceral adipose tissue of obese Otsuka Long-Evans Tokushima Fatty rats, an animal model of type 2 diabetes [17]. Li et al. (2008) found that vaspin levels are usually high in diabetic or insulin-resistant individuals compared to normal individuals with low weight [18]. Additionally, Wada (2008) concluded that vaspin has a modulatory role in glucose metabolism [19]. Castro et al., (2017) concluded that vaspin analogues or antagonists can modify insulin sensitivity in metabolic syndrome [20].According to our resources, we found no available study demonstrated the effect of sleeve gastrectomy on serum levels of kisspeptin, so this study is designated to demonstrate this effect and search the timing of this effect in relation to weight loss.
2. Materials and Methods
- Animals40 male albino rats (weight 200–220 g), provided by the Laboratory Animal house from Faculty of Medicine Zagazig University, were housed in a 12-h light/dark cycle under constant temperature (24 ± 2°C) and humidity (60 ± 10%) in independent ventilated cages. After being acclimated for 2 weeks, the weight, food intake, fasting glucose, serum Kisspeptin, Vaspin, Insulin, HOMA-IR of the rats were measured. This study was done in physiology department, Faculty of Medicine, Zagazig University Egypt. Chemicals Kisspeptin commercial enzyme immunoassay (EIA) kits (Phoenix Pharmaceuticals Inc., Burlingame, California, USA) purchased from Sigma Aldrich Cairo Egypt.Vaspin commercial enzyme immunoassay (EIA) kits purchased from Sigma Aldrich Cairo Egypt.Induction of type 2 diabetic model30 rats (out of 40) were given access to clean water and a high-fat diet (HFD, 40% fat, Huafukang Biotech, China) for 1 month to induce insulin resistance and then were injected with streptozotocin (STZ, 35 mg/kg) (Sigma, USA) intraperitoneally. After induction of diabetes (rats with random blood glucose ≥16.6 mmol/l (≥ 300 mg/dl) are divided into 3 groups:2nd group Dioabetic (D) without procedures3rd group Diabetic undergone Sham Operation (DSO).4th group diabetic undergone Sleeve gastrectomy (DSG) So as total we have four groups: 1st group: control (C) group (n=10).2nd group: Diabetic (D) rats without procedures (n=10). 3rd group: Diabetic rats with Sham Operation (DSO) (n=10). 4th group: Diabetic rats with Sleeve Gastrectomy (DSG) (n=10) Surgical Procedures: Before each procedure, rats were fed 10% Ensure (Abbott, USA) for 2 days and then fasted for 12 h. Sleeve Gatrectomy [21, 22]: rats were anesthetized with intraperitoneal injection of 10% chloral hydrate (3 ml/kg) before procedure. An upper abdominal incision of approximately 5 cm was performed and the gastric omentum and lesser omentum were then dissected. After ligation and transection of the gastric omental vessels in the pylorus area, we used forceps to clamp the greater curvature in case of hemorrhage. The portion of the stomach outside the clamped area, which was approximately 70% of the whole stomach volume and included the gastric fundus, was resected. The stomach incision was sutured with 5-0 silk suture (Ningbo Medical Needle, China). The abdomen was closed after leakage and hemorrhage was prevented. Sham Operation [23]: A laparotomy was performed to expose the stomach and esophagus and operative time was prolonged to mimic that experienced by the SG rats. Subsequently, the abdominal incision was closed.Postoperative CareAt the end of the surgical procedures, all rats received sterile 0.9% NaCl 10 mL i.p. and 10 mL s.c. to maintain hydration during healing. The animals received ketoprofen 5 mg/kg as an analgesic. They were placed on a heated mat until they recovered and then were returned to their home cages. The rats were allowed to drink purified water for 12 h after surgery, and a liquid diet containing 5% glucose and 0.2% KCl was provided for the next 48 h. Thereafter, they received the HFD until 10 weeks after surgery.Body Weight, fasting glucose, plasma insulin and HOMA-IR were measured in all groups and in SO and SG groups at the 2nd, 4th and 10th week after the surgery. Also, serum levels of Vaspin, Kisspeptin are measured in all groups. Food intake was calculated by the difference in weight between the offered diet and the weight of the rest of the diet. Blood glucose by samples taken from the rat tail vein.Biochemical analysesSerum was obtained by centrifugation of blood sample. Serum kisspeptin levels were measured by a commercial enzyme immunoassay (EIA) kit (Phoenix Pharmaceuticals Inc., Burlingame, California, USA). The range of kisspeptin was 0-100 and the minimum detectable concentrations were 0.06 ng/ml. Serum vaspin was measured using the enzyme-linked immunosorbent assay (ELISA) kit. Vaspin values were obtained with Rat EIA-VAP (RayBiotech_, Norcross, GA, USA). Plasma glucose levels were analyzed by the glucose oxidase method (Glucose Analyzer II; Beckman Coulter, Fullerton, CA). Serum insulin was measured by a rat insulin ultrasensitive ELISA (BioVendor, Kassel, Germany). Homeostatic model assessment for insulin resistance (HOMA-IR) was calculated [24].Statistical Analysis: GraphPad QuickCalcs software was used for calculation of unpaired t-test. The data obtained from serological examination of kisspeptin was expressed as mean values ± standard Error of Mean (SEM). Statistical analysis was performed in unpaired t-tests. P value ≤ 0.05 was taken to indicate statistical significance. Pearson Correlation coefficient between Body weight and serum Kisspeptin was calculated by Social Science Statstics software.
3. Results
- Table 1 showed that body weight and food intake increased significantly in diabetic rats in comparison with the control group (310± 30, 200±20 respectively, p=0.006) (200±3, 150±6 respectively, p=0.0001). Insignificant changes in body weight are found in diabetic sham group at 2w, 4w and 10 weeks in comparison with diabetic group (307±23, 306±22, 306±20 and 310±30 respectively, p=0.9). Also, insignificant changes in body weight are found in diabetic rats with sleeve gastrectomy after 2 weeks in comparison with diabetic group (305±25 and 310±30 respectively, p=0.8) however, significant and highly significant reduction in body weight were found in diabetic rats with sleeve gastrectomy after 4 and 10 weeks in comparison with diabetic group (237±16, 201±21 and 310±30 respectively, p=0.04 and 0.008 at 4 and 10 W). Food intake was reduced significantly in Diabetic sham group at 2 w, 4 w and 10 weeks in comparison with the diabetic group (180±5, 179±7, 178±9 and 200±3 respectively, p=0.008). Highly significant reductions in food intake were found in diabetic group with sleeve gastrectomy at 2 w, 4 w and 10 weeks in comparison with the diabetic group (90±4, 105±5, 130±7 and 200±3 respectively, p=0.0001).Kisspeptin decreased significantly in diabetic rats in comparison with the control group (1.31±0.21, 2.11±0.31 respectively, p=0.04), however insignificant changes were found in sham diabetic group at 2 w, 4 w and 10 weeks in comparison with the diabetic group (1.11±0.29, 1.10±0.28, 1.12±0.27 and 1.31±0.21 respectively, p= 0.5). Extremely significant increases were found in Kisspeptin in diabetic rats with sleeve gastrectomy after 2w, 4 w and 10 weeks in comparison with the diabetic group (2.97±0.22, 3.43±0.23, 3.91±0.30 and 1.31±0.21 respectively, p=0.0001). significant, highly significant and extremely highly significant increases were found in kisspeptin srum levels in diabetic rats with sleeve gastrectomy after 2w, 4 w and 10 weeks in comparison with the control group (2.97±0.22, 3.43±0.23, 3.91±0.30 and 2.11±0.31 respectively, p=0.03, 0.003, 0.0006 respectively).Vaspin was decreased significantly in diabetic rats in relation to control (from 700±40 to 300±50, p=0.0001), however insignificant changes in serum vaspin were found in sham diabetic group at 2 w, 4 w and 10 weeks in comparison with the diabetic group (295±45, 296±44, 294±43 and 300±50 respectively, p= 0.9). Highly significant and Extremely highly significant increase in serum vaspin were found in diabetic rats with sleeve gastrectomy after 2w, 4 w and 10 weeks in comparison with the diabetic group (590±43, 670±50, 720±40 and 300±50 respectively, p= 0.0003, 0.0001).
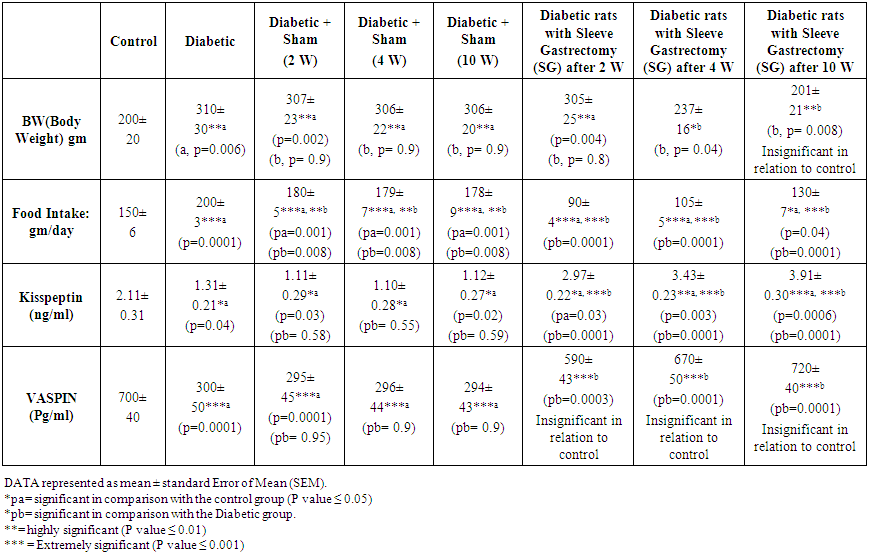 | Table 1. Effect of Sleeve Gastrectomy on Body Weight, Food Intake, Serum Kisspeptin and Vaspin |
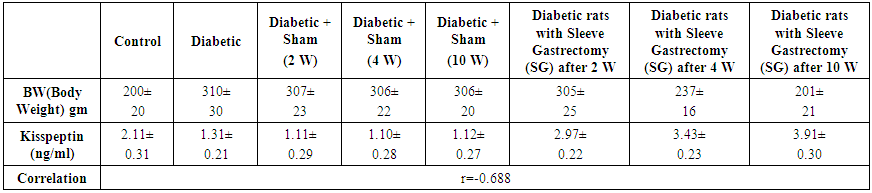 | Table 2. Correlation between Body Weight and serum Kisspeptin |
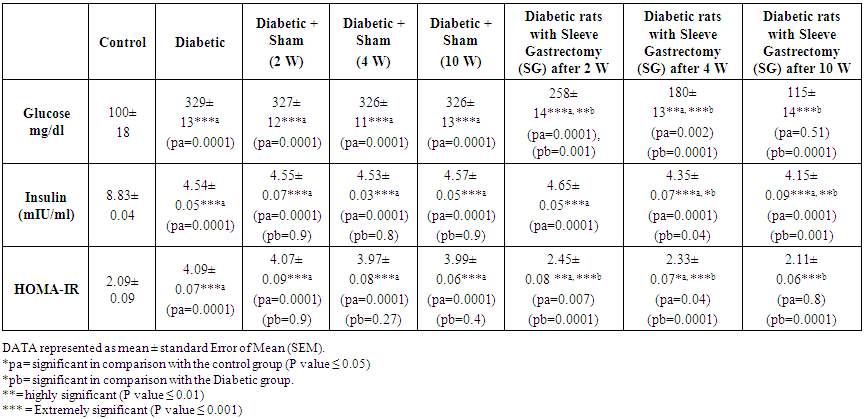 | Table 3. Effect of Sleeve Gastrectomy on Glucostatic Parameters |
4. Discussion
- Kisspeptin is now considered an important regulator of reproductive functions as it is involved in the direct activation of gonadotropin-releasing hormone (GnRH) [36]. Ohtaki et al., (2001) found that Kiss exhibited the ability to suppress tumor metastasis and they reported that high level of Kiss1 expression was found in healthy and tumorous human tissues [37]. According to our resources, we found no available study demonstrated the effect of sleeve gastrectomy on serum levels of kisspeptin, so this study is designated to demonstrate this effect and search the timing of this effect in relation to weight loss.In the present study, body weight and food intake increased significantly in diabetic rats in comparison with the control group (p=0.006 and 0.0001 respectively). Insignificant changes in body weight are found in DSG group at 2w, 4w and 10 weeks in comparison with D group (p=0.9). Also, insignificant changes in body weight are found in DSO group after 2 weeks in comparison with D group (p=0.8) however, significant and highly significant reductions in body weight were found in DSO group after 4 and 10 weeks in comparison with D group (p=0.04 and 0.008 at 4 and 10 W respectively). These findings are supported by Lombardo et al., (2010) who found that sleeve gastrectomy reduced body mass index from 58.2 to 44.5 Kg/m2 and they concluded that sleeeve gastrectomy is a safe and effective treatment for the high-risk and super-obese patient [15]. The findings of the present study suggest that body weight is affected late by sleeve gastrectomy after 4 and 10 weeks. Our suggestion is supported by Wang et al., (2017) who found that the SG group displayed significant weight loss 6 weeks postoperatively. In the present study, food intake was reduced significantly in DSO group at 2 w, 4 w and 10 weeks in comparison with D group (p=0.008). Highly significant reductions in food intake were found in DSG group at 2 w, 4 w and 10 weeks in comparison with D group (p=0.0001). These findings are in agreement with Wang et al., (2017) who found that the Sleeve Gastrectomy group displayed significant reduction in food intake 4 weeks postoperatively [25]. In the present study, Kisspeptin decreased significantly in D group in comparison with the C group (p=0.04), however insignificant changes were found in DSO group at 2 w, 4 w and 10 weeks in comparison with D group (p= 0.5). These findings are in agreement with Dudek et al., (2016) who found that diabetic rats have changes in Kiss1 and/or GPR54 mRNA levels in the hypothalamic-pituitary-gonadal axis as well as in peripheral tissues [26]. The findings of the present study are also supported by Zhou et al., (2014) who found A marked suppression of ovarian Kiss1 mRNA levels in high fat diet (HFD) rats compared with the normal chow diet controls. They concluded that exposure of female rats to a high-fat diet may involve down-regulation of ovarian Kiss1 mRNA and kisspeptin [27]. In this study, Significant, highly significant and extremely highly significant increases were found in kisspeptin serum levels in DSG after 2w, 4 w and 10 weeks in comparison with C group (2.97±0.22, 3.43±0.23, 3.91±0.30 and 2.11±0.31 respectively, p=0.03, 0.003, 0.0006 respectively). Extremely significant increases were found in Kisspeptin in DSG after 2w, 4 w and 10 weeks in comparison with the D group (2.97±0.22, 3.43±0.23, 3.91±0.30 and 1.31±0.21 respectively, p=0.0001). This significant increase in kisspeptin serum levels in DSG group might be due to sudden drop in serum Ghrelin levels after gastrectomy as ghrelin is a strong inhibitor of kisspeptin. This explanation is supported by Sagheb et al., (2017) who found that Ghrelin (10-6M) significantly decreased the transcription of Kiss-1 compared to the C group in the islets of Langerhans and this concentration of ghrelin significantly diminished KissR transcription in islet cells too. They also found that ghrelin has a significant inhibitory effect on KiSS-1 and KissR mRNA transcription in CRI-D2 cells (insulinoma Cell line) [10]. Our explanation was also supported by Zhu et al., (2014) who found that ghrelin secretion of Sleeve Gastrectomy group was significantly decreased (P < 0.005) [31]. In the present study, Vaspin was decreased significantly in D rats in relation to C group (from 700±40 to 300±50, p=0.0001), however insignificant changes in serum vaspin were found in DSO at 2 w, 4 w and 10 weeks in comparison with the D group (295±45, 296±44, 294±43 and 300±50 respectively, p= 0.9). These findings are supported by the findings of Castro et al., (2017) who found that Vaspin level was lower for the D group than for the Non-D group [20]. Also the present study in agreement with Li et al. (2008) [18] who suggested that vaspin might have an insulin-sensitizing effect mainly on white adipose tissue. However, these findings are in controversy with Li et al. (2008) in their report that vaspin levels are usually high in diabetic or insulin-resistant individuals compared to normal individuals with low weight [18], this controversy may be due to species differences where this study was carried out on albino rats and also study of Castro et al (2017) on wister rats however Lie et al (2008) study was conducted in human. The present study is also supported by Hida et al. (2005) [17] who found that vaspin levels were markedly reduced in OLETF rats, an animal model of type 2 diabetes, when they developed severe hyperglycaemia at 50 weeks.In this study, highly significant and extremely highly significant increase in serum vaspin levels were found in DSG group after 2w, 4 w and 10 weeks in comparison with the D group (590±43, 670±50, 720±40 and 300±50 respectively, p= 0.0003, 0.0001). These findings are in agreement with Tomasz., (2015) who found that vaspin serum level was significantly higher after ileal transposition in relation to normal control rats [28]. In the current study, moderate negative correlation (r= - 0.688) was found between serum Kisspeptin and body wight in all groups which means that Kisspeptin increases associated with decrease in Body Weight however, this associaten is incomplete and there is a tendency for Kisspeptin to increase without corresponding decrease in body wight. Extremely significant increases were found in Kisspeptin in DSG groups after 2w, 4 w and 10 weeks in comparison with the D group (2.97±0.22, 3.43±0.23, 3.91±0.30 and 1.31±0.21 respectively, p=0.0001) without corresponding or associated significant decrease in body wight at 2 weeks in comparison with D group (305± 25 and 310± 30 respectively, p=0.8). Body wight reduction become significant after 4 weeks and highly significant after 10 weeks in comparison with D group (237±16, 201±21 and 310± 30 respectively, p=0.04 and 0.008 at 4 and 10 W). The highly significant increase in Kisspeptin at week 2 with insignificant body wight reduction indicates early changes in serum kisspeptin after sleeve gastrectomy preceding weight changes. Our findings and explanation were supported by Pories et al., [33] who reported that the glycemic control often occurs within days before significant weight loss has been reached. Also, our explanation was supported by Zhu et al., (2014) who suggested that the control of the glycemic status may be a direct effect of surgery rather than a secondary effect of weight loss [31].In the present sudy, extremely significant increase in serum Glucose in D, DSO groups at 2w, 4w, and 10 weeks in comparison with the C group (p= 0.0001). Highly significant and very highly significant reductions was found in glucose levels in DSG after 2w, 4 w and 10 weeks in comparison with the D group (258±14, 180±13, 115±14 and 329±13 respectively, p=0.001, 0.0001). The present study was supported by Nosso et al., (2011) who found that sleeve gastrectomy is effective in producing a significant and sustained weight loss and improving glucose homeostasis in severely obese T2DM patients [30]. The present study was also supported by Zhu et al., (2014) who found that fasting blood glucose of the rats in the SG group had significantly decreased with the improved glucose tolerance [31]. Insignificant changes are found in glucose level in DSG after 10 weeks in comparison with the C group (115±14 and 100±18 respectively, p= 0.51) which suggest improved serum Glucose level and return to semi-normal. This finding and suggestion is supported by Liu et al., (2017) who reported that sleeve gastrectomy resulted in better glucose tolerance, lower HOMA-IR, up-regulated hepatic insulin signaling [29]. In the current study, Serum insulin was decreased significantly in D and DSO groups (2w, 4w and 10 w) in relation to C group (p= 0.0001). Insignificant changes in serum insulin were found in DSO groups (2w, 4w and 10 w) in relation to D group (p=0.9). Insignificant changes are also found in serum insulin levels between DSG after 2 weeks in comparison with the D group (4.65±0.05 and 4.54±0.05 respectively, p=0.3). Significant and highly significant reduction in serum insulin levels in DSG after 4 w and 10 weeks in comparison with the D group (4.35±0.07, 4.15±0.09 and 4.54±0.05 respectively, p=0.04, 0.001 respectively). Significant reductions are found in serum insulin levels between DSG after 2w, 4 w and 10 weeks in comparison with the C group (4.65±0.05, 4.35±0.07, 4.15±0.09 and 8.83±0.04 respectively, p= 0.0001). These findings are supported by Lee et al., (2010) who found that the mean fasting plasma insulin levels were reduced significantly from 16.8± 15.4 to 5.6±3.2 uIU/mL at 1 week after operation [34]. The present study was also supported by Silvestre et al., (2008) who found that Kisspeptin-13 reduced glucose-induced insulin secretion in a dose-dependent manner and inhibited the insulin responses to both carbachol and exendin-4 and they concluded that kisspeptins may be implicated in the regulation of B-cell [35].The present study found that the HOMA-IR was significantly increased in D and DSO (2w, 4w and 10 w) groups in comparison with the C group (p=0.0001). No significant changes were found in DSO (2w, 4w and 10 w) groups in comparison with the D group (4.07±0.09, 3.97±0.08, 3.99±0.06 and 4.09±0.07, p=0.9, 0.27 and 0.4 respectively). Highly significant and significant increase in HOMA-IR in DSG groups after 2w and 4 weeks in relation to C group (2.45±0.08, 2.33±0.07 and 2.09±0.09 respectively, p=0.007 and 0.04 respectively) however, no significant change was found between DSG group after 10 w and the C group (2.11±0.06 and 2.09±0.09 respectively, p=0.8). Very highly significant reductions in HOMA-IR in DSG at 2w, 4 w and 10w in relation to D group (2.45±0.08, 2.33±0.07, 2.11±0.06 and 4.09±0.07, p= 0.0001). These findings are in agreement with Basso et al., (2016) who found that Insulin sensitivity was significantly improved in sleeve gastrectomy compared with Sham operated rats as demonstrated by HOMA-IR values, which were reduced by ∼50% (p<0.0001) [32]. However, the present study is in controversy with Basso et al., (2016) in that they found significant reduction in insulin level rather than significant increase, this controversy may be explained by the model used in their study which is vertical sleeve gastrectomy in which the fundus of stomach is left intact.
5. Conclusions
- In this study, Significant increases were found in kisspeptin and Vaspin serum levels in diabetic rats with sleeve gastrectomy after 2w, 4 w and 10 weeks in comparison with C and D groups (p≤0.05). This significant increase in kisspeptin srum levels in diabetic rats with sleeve gastrectomy might be due to sudden drop in serum ghrelin levels after gastrectomy as ghrelin is a strong inhibitor of kisspeptin. A moderate negative correlation (r= - 0.688) was found between serum Kisspeptin and body wight in all groups which means incomplete associaten and there is a tendency for Kisspeptin to increase without corresponding decrease in body wight. Extremely significant increases were found in Kisspeptin in diabetic rats with sleeve gastrectomy after 2w, 4 w and 10 weeks in comparison with the D group (p=0.0001) without corresponding or associated significant reduction in body wight at 2 weeks in comparison with diabetic group (p=0.8). Body wight reduction become significant after 4 weeks and highly significant after 10 weeks in comparison with diabetic group (p=0.04 and 0.008 at 4 and 10 W). The highly significant increase in Kisspeptin at week 2 with insignificant body wight reduction indicates early changes in serum kisspeptin after sleeve gastrectomy preceding weight changes.
6. Recommendations
- The role of kisspeptin in reducing weight should be investigated in humans and this role could serve in treatment of obesity as alternative to surgery. Drugs acting on kisspeptin receptors should be investigated in further studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML