-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2017; 6(3): 47-53
doi:10.5923/j.diabetes.20170603.01

The Effect of Twelve Weeks Supervised Aerobic Exercise Intervention on Lower Extremities Oxygenation and Wound Healing among Diabetic Ulcer Subjects
Maduabuchi Joseph Nwankwo1, Goddy Chuba Okoye2, Afamefuna Victor Egwuonwu1, Antoninus Obinna Ezeukwu2
1Department of Medical Rehabilitation, Nnamdi Azikiwe University, Nnewi Campus, Nigeria
2Department of Medical Rehabilitation, University of Nigeria, Enugu Campus, Nigeria
Correspondence to: Afamefuna Victor Egwuonwu, Department of Medical Rehabilitation, Nnamdi Azikiwe University, Nnewi Campus, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Diabetic foot ulcer is associated with peripheral neuropathy due to hyperglycemic state of the individuals, leading to low tissue oxygen perfusion. There is paucity of substantial evidence on the role of aerobic exercise on vascular blood perfusion and capillary oxygen tension associated with wound healing among diabetic foot ulcer patients. Methods: The study was a pretest-posttest randomized control trial design that consecutively recruited 61 diabetic foot ulcer subject, from the out-patient clinic of Nnamdi Azikiwe University Teaching hospital, Nnewi. The subjects after warm-up exercise rode on bicycle ergometer exercise at 60% of their maximum heart rate achieved on stress test and progressed to 85% of heart rate maximum. The participants were randomly assigned into exercise and control groups, and tested for oxygen percentage saturation and ankle brachial index at baseline and every 2 weeks for 12 weeks. The subjects had an ulcer of at least 1cm2 area with minimum of 30 days duration. Results: There was a significance difference in the oxygen percentage saturation and ankle brachial indexes between the two groups at the end of the intervention with p < 0.05. There was also a significant differences in the percentage wound size reduction for the two groups with a p < 0.05 post intervention. There was a significant correlation between oxygen percentage saturation, ankle brachial index and duration of diabetes with p < 0.05 after 12weeks. Conclusion: Aerobic exercise significantly increased oxygen percentage saturation, thereby resulting in wound healing in the experimental group at the end of the twelve weeks intervention. We recommend that aerobic exercise intervention should be an essential component of treatment plan for patients with diabetic foot ulcer.
Keywords: Oxygen percentage saturation, Ankle brachial indexes, Aerobic exercise
Cite this paper: Maduabuchi Joseph Nwankwo, Goddy Chuba Okoye, Afamefuna Victor Egwuonwu, Antoninus Obinna Ezeukwu, The Effect of Twelve Weeks Supervised Aerobic Exercise Intervention on Lower Extremities Oxygenation and Wound Healing among Diabetic Ulcer Subjects, International Journal of Diabetes Research, Vol. 6 No. 3, 2017, pp. 47-53. doi: 10.5923/j.diabetes.20170603.01.
Article Outline
1. Background of the Study
- Diabetes is a metabolic disorder resulting in chronic hyperglycemia and hyperlipidemia which eventually leads to diverse multiple system pathologies such as atherosclerosis, coronary heart disease, myocardial infarction, and stroke [1]. There are two major classifications of diabetes; Type 1 and Type 2. Type 1 diabetes, is primarily the result of the inability to produce insulin due to beta cell destruction in the pancreas and affects about 10-15% of all people with diabetes [2]. While type II diabetes, results from a combination of insufficient insulin production and/or resistance of the body cells insulin actions and affects over 80% of individuals diagnosed with diabetes, [3]. Diabetes is the third leading cause of death in Nigeria and affects about 6% of the world’s population [4, 5]. People living with diabetes have about 12% risk of developing foot ulcer at some point in their lifetime [6]. The most common complication of diabetes mellitus, results from macro vascular and micro vascular structural changes culminating into neuropathy in the lower extremity known as diabetic foot ulcer. Diabetic foot ulcer is associated with peripheral neuropathy or large vessel disease due to hyperglycemic state of the individuals, which overtime results in the irreversible covalent modification of structural protein and lipids (glycosylation), compromising the extracellular matrix, connective and vascular tissues [7]. These structural changes causes impairment of capillary function, poor blood tissue and organ perfusion and the release of oxidizing agents triggering systemic inflammatory process. Diabetic foot ulcerations is also associated with reduced nerve perfusion which is an important factor in the etiology of diabetic neuropathy, [8]. Accumulation of lipids inside blood vessels (atherosclerosis) can prevent blood supply to certain peripheral nerves [2]. Injuries that damage the microvasculature attract inflammatory cells that consume large amounts of oxygen and concentrate potentially damaging products at the wound site [9]. This creates a low-oxygen environment with low pH, high lactate, increased oxidant production and poor local tissue perfusion. This lack of oxygen and nutrients, to the surrounding tissues and nerves leads to ischemia and tissue necrosis. The inter-relationships of all these factors; contribute to formation of lesions which results to gangrene (tissue infection) and ultimately amputation [2]. Diabetic foot problems constitute the primary cause of hospitalization of people with diabetes globally [10]. Amputation is common and the statistics of diabetes related amputation in Nigeria is increasing, about 28% to 51% of diabetics in tertiary healthcare facilities undergo amputation [11]. There is a high probability that diabetics with previous history of amputation would need a centralized one within the next five years globally [8] The healthcare burden this large number of diabetics will place on healthcare services, are enormous even if a fraction will develop foot complications at any point in life [11].Under normal circumstances, endothelial cells are able to metabolize circulating blood glucose, providing the energy that the body needs to function [9]. However, in hyperglycemia, too many glucose molecules in the blood cause endothelial cells to malfunction, damaging the body’s circulatory system and resulting in serious complications that can range from kidney failure and heart disease, to blindness. Prolonged hyperglycemia usually results to death of the patient [8, 12].The endothelium has a unique ability to repair and regenerate itself with the help of a group of specialized cells called endothelial progenitor cells (EPCs) [13]. Endothelial progenitor cells are found in bone marrow and respond to signals from damaged endothelial tissue [8]. They are recruited into the bloodstream to help repair injured vessel. Endothelial progenitor cells can act both directly and indirectly at the site of blood vessel injury by integrating into vessel walls and releasing growth factors that help form new blood vessels at the site of the injury [13]. The endothelial progenitor cells of diabetics are exposed to high concentration of blood glucose that leads to malfunctioning, death and lowering of the plasma levels of these growth cells [8]. A continuous supply of oxygen to all tissues is necessary for the efficient production of ATP, and this supply is considered sufficient when aerobic metabolism is maintained. Non healing wounds, necrotizing infections, radiation-induced necrosis, crush injury, decompression illness, and carbon-monoxide poisoning all exhibit impaired tissue oxygenation [14]. The need for efficacy of an intervention that will promote tissue oxygenation in such conditions is in part determined by the prevailing state of tissue oxygen supply and demand [15]. The methods currently available or under development for assessing the adequacy of tissue oxygenation include blood gas analysis, transcutaneous pulse oximetry, gastric tonometry, near-infrared spectroscopy, functional magnetic resonance imaging, magnetic resonance spectroscopy, electron paramagnetic resonance, positron emission tomography, and single photon emission computed tomography [16]. Studies have also shown that most of these techniques further exposes the diabetic individuals to radiation risks and the sophistication of such procedure make it inaccessible and difficult to administer [16]. The use of transcutaneous pulse oximetry was chosen in the present study to measure lower extremities oxygen-tension because it is non-invasive, less sophisticated and without radiation risks to patients [14]. The transcutaneous pulse oximetry measures blood oxygen saturation level in plasma and this can be used to estimate lower extremities oxygen-tension [14].The plasma oxygen percentage saturation affects ankle brachial index estimate since poor oxygen percentage saturation in blood will lower blood pressure at the point with reduced tissue oxygen-tension [15]. Ankle brachial index (ABI) is a non-invasive vascular screening test to identify large vessel, peripheral arterial disease such as chronic lower extremity arterial disease (LEAD) which is a progressive disease [17]. Risk factors for LEAD are aging, smoking, diabetes, dyslipidemia, hypertension, hyperhomcysteinemia, renal insufficiency and family history of cardiovascular disease [18]. A decrease in ABI below 1- 0.5 simply means low blood perfusion and poor wound healing. However, an abnormal increase in ABI above 1.3 denotes false prediction of oxygen perfusion this is found in people with arterial calcification due to glucose and lipid accumulation around the ankle causing stiffness and incompressible vessels [17].In a previously related study published by [5] it was reported that supervised aerobic exercise intervention significantly reduced the wound size mostly after the fourth week. The study also observed that the plasma concentration of fasting blood glucose of diabetics was reduced with about three sessions of aerobic exercise of at least sixty minutes per week. Further evidence to substantiate the effect of interventions designed to enhance the healing of chronic ulcers is urgently needed using the lower extremities oxygen percentage saturation and by extension ankle brachial index [7]. Meanwhile, exercise for decades has been considered a cornerstone of diabetes management, along with diet and medication. Exercise has both positive and negative effects on the perfusion of lower limbs with peripheral arterial occlusive disease (PAOD). The need to improve endothelial functions is paramount for diabetic individuals and there is evidence that exercise will result in total enhancement of blood circulation by lowering the plasma glucose concentration [17]. Despite conservative treatment of diabetic ulcers with multiple standard interventions such as surgical debridement, relief of pressure, many diabetic foot ulcers persist as non-healing wounds, eventually leading to limb amputation [19]. Despite substantial evidence on the role of aerobic exercise on vascular blood perfusion and capillary oxygen tension, there was paucity of study that have associated increased vascular blood perfusion with wound healing among diabetic foot ulcer patients, necessitating that we further analyze the results from our previously published study [5].
2. Methods and Materials
- SubjectsThe subjects were known type 1 or type 2 diabetic mellitus patient. Informed consent were obtained from the participants prior to the study and medical history which include basal heart rate, blood pressure, date of diabetes mellitus diagnosis, medication and co morbidities were collected. The pre-exercise stress test was arranged to ensure clearance for participation. All subjects had normal cognition as determined by a Folstein mini-mental status examination score of >23. The study was a twelve weeks supervised aerobic exercise program, that involved sixty one accessible adults with mean age of 69±4.79, that participated in the study, they were randomly assigned into two groups using the pitcher bowl method, group one (experimental group) are 31 diabetics with foot ulcers (16 males and 15 females), group two (non-experimental group) are diabetic with foot ulcers (15 males and 15 females) attending the diabetic clinic of Nnamdi Azikiwe University Teaching Hospital, Nnewi.Inclusion CriteriaThey were recruited for the study based on meeting certain inclusion criteria which is as follows: eligible subjects were sedentary for at least 6 months prior to the study. All the subjects had diabetic foot ulcers as a result of type 1 or 2 with at least 1cm2 (greatest length x greatest width) and at least foot ulcer of 30 days duration. The protocol was designed according to the fundamental treatment principal of the expert panel to the 2004 American Diabetic Association [20] consensus development conference on diabetic foot wound care.Exclusion CriteriaSubjects with congestive heart failure, uncontrollable cardiac arrhythmias, severe valvular heart diseases, individuals with uncontrolled BP (systolic BP>165/mmHg), extreme claustrophobia, Hematological disease that affects mobility, impaired knee flexion of <1900 and severe illness that precluded them from exercising, were excluded from the study.Study DesignThe study was a pretest-posttest randomized control trial design without single or double blinding of the participants.Sampling TechniqueThe subjects were recruited using purposive non-probability sampling technique.InterventionSubjects reported to the exercise clinic 3times a week, the two groups were assessed for baseline data which include height (using stadiometer), weight (using Hana bathroom scale, wound size area measurement was done for the two groups using transparent ruler to assess the wounds greatest length and width after which the wound size was calculated by multiplying the greatest length and width in centimetres. The follow up evaluations and wound size assessment were done on a 2 weekly basis for the twelve weeks study period. If a subject pre-exercise blood pressure is >165/90mmHg, he was asked to rest for 10minutes and reassessed. No exercise was permitted that day if a lower resting blood pressure was not achieved. Subjects were tested for cholesterol level and fasting blood sugar level, if the sugar level was less than 100mg/dl. They were giving a chance to take a 15gm carbohydrate snacks and reassessed 20minute later to ensure that the blood sugar level was not dropping below 100mg/dl before exercise intervention. The exercise precaution was based on recommendation by American Diabetes Association [20].Exercise ProtocolInitial aerobic exercise intensity was based on 60% of max. HR achieved on a stress test.Each subject was progressed to 85% of the value over 12weeks. All subjects were engaged in an aerobic warm-up of at least 5minutes with perceived rating in the light range of Borg’s rating of perceived exercise scale, following the warm up, the subjects were instructed to start with a ten minutes exercise which was increased until the exercise intensity gets to the range of target heart rate and rate of perceived exertion (RPE) to commensurate with “somewhat hard” (4 point), HR and RPE were monitored to ensure subjects were exercising at their prescribed intensity throughout the study. Subjects were encouraged to increase their exercise time by 5minute each two weeks until they reach 50minute at the ninth week, which was maintained until the end of the 12weeks program.Each of the subjects were exercising under supervision throughout the study and rode on a bicycle ergo meter with foot interaction kept constant with a standard gym pedal and a specialized off-loading insole padding to relieve pressure on the ulcer. Instrumentation1. Stop watch model Heuer tracmate brand was used for time measurement2. Transparent ruler made in China was used to measure the greatest length and width of the wound3. Stadiometer model Butterfly brand made in China was used to measure subject’s height4. Bicycle Ergometer model (PRO-ACTING Model) was used for non-weight bearing aerobic exercise5. Bathroom weighing scale Hana brand made in China was used to measure subject’s weight6. Borg’s rating scale of perceived exertion was used to monitor subject’s level perceived exertion during exercise. It is an open ended scale from 0 (Resting) to 10 (Very very hard).7. Folstein mini mental scale was used to assess subject’s cognition.
3. Results
- A total of sixty one participants were involved in the study and the social-demographics were similar to the previous publication by [5]. The summary of the findings from previous publication by [5] shows that the plasma glucose level significantly reduced mostly at the end of the fourth week of aerobic exercise intervention. There was also significant decrease in wound size area at the end of the twelve weeks intervention signifying healing of diabetic foot ulcer among subjects attributable to the aerobic exercise. The present article was undertaken to further analyze the findings of the study in terms of the association between oxygen percent saturation and ankle brachial indices with percentage wound size reduction between the two study groups.
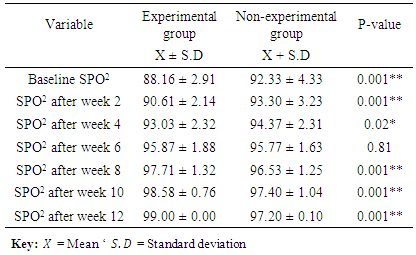
3.1. Comparison of Oxygen Percentage Saturation (SPO2) between the Two Groups
- The result from Table 1 showed that the baseline oxygen percentage saturation for both groups were 88.16 ± 2.91 (experimental) and 92.33±4.33 (non-experimental). While, the oxygen percentage saturation at the end of twelve weeks were 99.00±0.00% and 97.20±0.10% for experimental and non-experimental groups respectively. There was a significance difference in the oxygen percentage saturation between the two groups at the end of the study with a p-value of 0.001**.
|
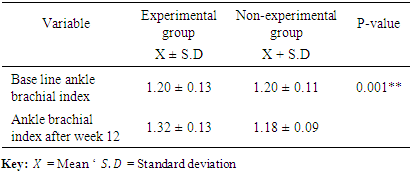
3.2. Comparison of the Ankle Brachial Index of the Two Groups
- The result from table 2 showed that the baseline Ankle brachial indexes for both groups were 1.20±0.13 and 1.20 ± 0.11 for experimental and non-experimental groups respectively. While the ankle brachial index at the end of twelve weeks were 1.32±0.13 and 1.18 ± 0.09 for experimental and non-experimental groups respectively. This shows a significant difference in the ankle brachial indexes for the two groups with a p-value of 0.001**. Table 2
|
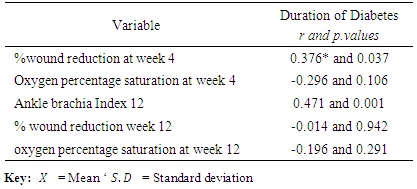
3.3. Relationship of Duration of Diabetes with Percentage Wound Size Reduction, Oxygen Percentage Saturation and Ankle Brachial Index
- The results showed that there were no significant correlation between duration of diabetes and percentage wound size reduction with r =-0.014; p value = 6.942, between oxygen percentage saturation and duration of diabetes after four weeks with r =-0.296; p value 0.106 and at the end of twelve weeks with r =-0.196; p value = 0.291. Meanwhile, there was a significant correlation between Ankle brachial index and duration of diabetes with r =0.471; p value = 0.001*after 12weeks and percentage wound size reduction after four weeks with r = 0.376; p value = 0.03**. (Table 3).
|
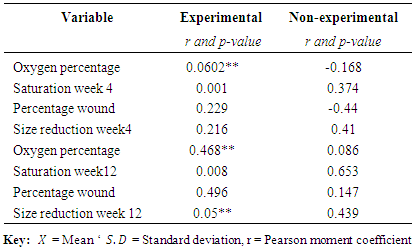
3.4 Relationship between BMI, Oxygen Percentage Saturation and Percentage Wound Size Reduction
- The result from table 4 showed that there were significant correlation between BMI and oxygen percentage saturation after four weeks of intervention, there was no significant correlation between BMI and percentage wound size reduction after four weeks, (r=0.0602**; p=0.000** and r=0.229; p= 0.216) respectively for the experimental group. While there were no significant correlation between BMI and oxygen percentage saturation after four weeks for the non-experimental group, (r=-0.168; p=0.374 and r=-0.44; p= 0.41). Additionally, after the twelve weeks intervention the result showed that there was a significant correlation between BMI and percentage wound size reduction and oxygen percentage saturation in the experimental group, (r=0.468**; p=0.008** and r=0.496*; p= 0.005), but there was no significant correlation in the non-experimental group between BMI, percentage wound reduction and oxygen percentage saturation, (r=0.086; p=0.653 and r=0.147; p= 0.439) respectively.
|
4. Discussion
- The results of the present study showed that the baseline oxygen percentage saturation in the experimental group (88.16±2.91) was lower compared to that of the non-experimental group (92.33±4.33) this may imply that the subjects in the experimental group had longer duration of diabetes and larger wound size area at baseline. However, there was a sharp contrast seen at the end of the 12 weeks aerobic exercise intervention with the experimental group recording a rise in oxygen percentage saturation compared to the baseline which may be a sign of improved peripheral blood oxygen (99.00±00) compared to 97.20±0.10 in the non-experimental group, despite the longer duration of diabetes of the experimental group. The implication of this finding is that aerobic exercise intervention had a positive effect on the oxygen percentage saturation in the lower extremities of the subjects. This is similar with the findings of [21] that noted that diabetic foot ulcer patients suffers from disturbed circulation although there study could not provide evidence of peripheral vascular disease and could not associate the disturbed circulation to a decrease in oxygen supply of the foot. It is a fundamental clinical observation that wounds that do not bleed has slow healing and wounds that bleeds extensively heal faster. Continuous supply of oxygen to tissue through microcirculation is vital for the healing process and for resistance to infection [10]. However, oxygen is important for successful wound healing due to the increased demand for reparative process such as cell proliferation, bacterial defense, angiogenesis and collagen synthesis [22]. There was also a significant difference in oxygen percentage saturation between the experimental group and control group with a p-value of 0.001** at 95% CI. This agrees with the findings of a study by [23] that noted a significant improvement in oxygen perfusion with exercise intervention. However, this was in contrast with the findings of [24] that reported that aerobic exercise had no significant effect on tissue oxygen perfusion in a similar study conducted in Egypt among type II diabetic subjects. Improvement in oxygen percentage saturation was recommended previously as an accurate and reliable means of predicting wound healings in patients with diabetic ulcers [25].Additionally there is evidence that the increase in oxygen percentage saturation recorded in the present study may be associated with the aerobic exercise intervention. It may also be responsible for the foot ulcers healing seen among the experimental group diabetic subjects. It is recommended that non-weight bearing exercise should be made a corner stone in the management of patients with diabetic ulcers [17, 5].The findings of the present study also found that there was a significant correlation between Ankle brachial index and oxygen percentage saturation at the end of the fourth, even though the wound size area was not significantly correlated with ankle brachial index. This is in contrast with the findings of [25] but agreed with that of [17] that also reported significant correlation between Ankle brachial indices with oxygen percentage saturation at the end of four weeks intervention. It is also a known fact that exercise usually has the greatest physiological effect mostly after the fourth week, [26]. Aerobic exercise enhances blood supply to contracting muscles (in these instance lower limbs), by causing vasodilatation of the surrounding vessels this may be responsible for increased ankle brachial index, observed in the experimental group compared to the control group. Meanwhile, in the non-experimental group continuous decrease in ABI was observed, which may be due to lack of adequate aerobic activity (1.18± 0.09) making the diabetic foot ulcer to heal slowly. More so, increases in ankle brachial indices implies enhanced oxygen supply to the extremities and nutrient, consequently increasing percentage wound size reduction among the subjects. However, abnormal increases in ABI above 1.3 may denote false prediction of oxygen supply this is found in people with incompressible vessel such as in calcification. The result of the present study showed that after four weeks of exercise intervention, the wound size areas significantly correlated with percentage wound size reduction with r and p value of (0.719** and 0.00) while there were no significant correlation between wound size area and oxygen percentage saturation after four weeks and this however agrees with the findings of [27] and [23]. This simply means that with conservative treatment of diabetic foot ulcers there is usually a marginal reduction in wound size taking place as observed in the non experimental group but with aerobic exercise as an adjunct intervention the wound size reduction rate doubles as in the experimental group. The study showed that percentage wound size reductions were higher during the fourth week of aerobic exercise intervention (49.07 ± 12.66) compared to the other weeks, which is similar to the findings of [23]. The present study also showed that duration of diabetes was significantly correlated with percentage wound size reduction at the end of fourth week exercise intervention in the experimental group but was not correlated with oxygen percentage saturation and ankle brachial index. This is in line with the report of [21] but contrasted with that of [25] that reported a significant correlation between, duration of diabetics with ankle brachial index and oxygen percentage saturation. The study also noted that percentage wound size reduction progressively increased in the experimental group from baseline to the end of the intervention, which was also significantly higher than that of the non-experimental group, the p-value 0.02**. It is possible also that chronic ulcer in diabetic subjects may mean slow or no healing at all. The duration of diabetes were found to be significantly correlated with percentage wound size reduction at fourth week. The findings were in line with the study of [23] and that of [17] but contrasted with the findings of [27] that conducted a study in Indonesia on the effect of exercise among diabetic neuropathic ulcer patients. The result also showed that there were significant differences in the ankle brachial index of the two groups which were reliable predictors of wound healing in diabetic foot ulcer patients as recommended by [17]. The normal ABI range is from 0.9 to 1.4, as the condition is getting worst the ABI drops. It was found out that there were significant negative correlation between the ABI and percentage wound size reduction (r = 0.397 and p = 0.02*). This implies that as the ankle brachial index was decreasing the percentage wound size reduction was increasing. The results of the present study showed that BMI was significantly correlated with oxygen percentage saturation at the end of twelve weeks but fail to correlate with percentage wound size reduction after twelve weeks intervention. This is in contrast with findings of both [23] and [27] that reported significant correlation between BMI, oxygen percentage saturation and percentage wound size reduction. The correlation of BMI with oxygen percentage saturation in the present study implies that diabetic foot ulcer subjects with high BMI may have slow healing response to aerobic exercise.
5. Conclusions
- Aerobic exercise significantly increased oxygen percentage saturation, thereby resulting in wound healing in the experimental group at the end of the twelve weeks intervention. The ABI, significantly correlated with oxygen percentage saturation mostly at fourth week, therefore may be a useful tool to predict improvement in oxygen percentage saturation, it is also indicative of the fact that aerobic exercise had its greatest effect on the wounds at about fourth week of intervention. It is recommended that aerobic exercise training should be an essential component of any treatment plan for patients with diabetic foot ulcer.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML