-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2017; 6(2): 41-46
doi:10.5923/j.diabetes.20170602.02

Circulating miR-155 Levels Correlate with Serum Neutrophil Gelatinase Associated Lipocalin in Patients with Diabetic Nephropathy
Khalid Mohammad Mahmoud Mohany1, 2, Mohamad Yosof Rezk3, Hany Ahmed Elkatawy3
1Basic Medical Science, Unaizah Collage of Medicine (UCM), Qassim University, Saudi Arabia
2Medical Biochemistry Department, Faculty of Medicine, Assiut University, Egypt
3Medical Physiology Department, Faculty of Medicine, Zagazig University, Egypt
Correspondence to: Mohamad Yosof Rezk, Medical Physiology Department, Faculty of Medicine, Zagazig University, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: diabetic nephropathy (DN) is a major cause of end-stage renal disease. The current study tested the circulating miR-155 levels and their correlation with serum neutrophil-gelatinase associated lipocalin (sNGAL), in normal persons and in patients with DN. Objectives: It aimed at finding a new biomarker for prediction DN to slowdown the loss of renal function. Subjects & Methods: this work is a case control study that included 48 participants, divided into: G1 [control, (n=10)] and G2, G3 and G4 [type 2 diabetic patients with normoalbuminuria (n=11), microalbuminuria (n=17), and macroalbuminuria (n=10), respectively]. Blood and urine samples were taken from all participants. Serum glucose, sNGAL, glycosylated hemoglobin percentage, urinary albumin were estimated by their specific kits, serum and urinary creatinine by alkaline picrate method and serum miR-155 values by RT-qPCR. Results: serum miR-155 and sNGAL levels differed significantly between groups (p=0.000), with high values observed in G4 followedby G3, G2 then G1. Significant high levels of miR-155 and sNGAL were found in G2 when compared to G1 (p= 0.013 and 0.0005 respectively). Serum miR-155 values correlated with sNGAL in G1 (r= 0.569, p= 0.009), G2 (r= 0.834, p= 0.000), G3 (r= 0.471, p= 0.004) and G4 (r= 0.596, p= 0.007). Conclusion: the miR-155 levels increase early in diabetic nephropathy even before the onset of microalbuminuria and these levels correlate with the sNGAL levels. Measurement of miR-155 levels could enhance the prediction of DN and improve its management protocol.
Keywords: Diabetic nephropathy (DN), miR-155, Serum neutrophil gelatinase associated lipocalin (sNGAL)
Cite this paper: Khalid Mohammad Mahmoud Mohany, Mohamad Yosof Rezk, Hany Ahmed Elkatawy, Circulating miR-155 Levels Correlate with Serum Neutrophil Gelatinase Associated Lipocalin in Patients with Diabetic Nephropathy, International Journal of Diabetes Research, Vol. 6 No. 2, 2017, pp. 41-46. doi: 10.5923/j.diabetes.20170602.02.
1. Introduction
- Diabetic nephropathy (DN) is a serious microvascular complication that is characterized by progressive albumin excretion in urine, increasing blood pressure, a decline in glomerular filtration and may end in renal failure [1, 2]. The pathogenesis of DN involves both glomerular dysfunction as well as tubulointerstitial damage [3]. Finding new biomarkers for early diagnosis and intervention of DN can slow down the loss of kidney function and improve patient outcomes. [4]Diabetic nephropathy can be predicted before the decline in the glomerular function by detecting the abnormal increase in amounts of urinary albumin (microalbuminuria, macroalbuminuria, and overt nephropathy) [5-7]. Also, the early detection of microalbuminuria in a diabetic patient can predict the risk for the development of ischemic heart [5].Serum neutrophil gelatinase–associated lipocalin (sNGAL) levels increased in both of type I and type II diabetic patients even before the onset of microalbuminuria [8]. Its measurement was suggested for the evaluation of early renal involvement in the course of diabetes [8, 9]. Neutrophil gelatinase–associated lipocalin (NGAL) is a 25-kDa protein belonging to the lipocalin proteins superfamily that is able to bind and transport small hydrophobic molecules [10]. Siderophores (iron-containing molecules), are the most common ligands of lipocalins [11]. After binding sidrophores, NGAL-sidrophore complex is internalized to the cell by a receptor mediated endocytosis to release its sedrophores iron content that initiates iron dependent specific pathways [12]. Two types of receptors have a role in NGAL endocytosis; 24p3R (mainly in brain) and the megalin multiscavenger complex (on the brush-border of renal tubular cells) [12, 13].microRNAs (miRNAs) are short non-coding RNA molecules (21–25 nucleotides) that affect cell cycle, apoptosis and metabolism. They are important in maintaining renal homeostasis and can modulate the expression of inflammatory mediators' genes in kidney diseases and may have a critical role in the development of DN [14], nephrolithiasis [15] and interstitial fibrosis and tubular atrophy in kidney transplantation [16].The present study compared the serum miRNA-155 (miR-155) levels between patients with diabetic nephropathy and healthy controls. Besides, it tested the correlation between serum miR-155 levels and sNGAL levels in these cases aiming at improving early diagnosis the treatment protocols.
2. Subjects and Methods
- The present study is a randomized case-control that was conducted in the Department Basic Medical Sciences, Unizah Collage of Medicine, Qassim University and Endocrinology and Diabetes Unit (outpatient clinic), King Saud Hospital between September, 2014 and June, 2015. The study was approved from the Unizah Medical School Ethical Review Board. A written informed consent was obtained and archived from all participants.The study included 48 participants (18 male and 30 female), aged between 35 and 70 years; they were grouped into:A. Cases: All consecutive patients (n=38) visited the Endocrinology and Diabetes Unit (outpatient clinic) and presented with type 2 diabetes mellitus for more than 5 years. They were treated either by oral hypoglycemic drugs or insulin therapy and divided according to urinary albumin to creatinine ratio (UA/CR) into 3 groups [19]; Normoalbuminuria group (UA/CR ratio is less than 20mg/gm, n= 11), Microalbuminuria group (UA/CR ratio is between 20 and 200mg/gm, n= 17) and Macroalbuminuria group (UA/CR ratio is more than 200 mg/gm, n= 10).B. Control: included 10 consecutive non diabetic healthy volunteers.Complete medical history, clinical examinations and routine hospital investigations were done for all participants. The participant was excluded from the current study when presented with any one of the following: type 1 diabetes mellitus, arterial hypertension, cardiovascular diseases, cancer, fever, infections, inflammatory status, leukocytosis, renal failure, hematuria, endocrinal disorder and other chronic debilitating diseases. From all participant 10mL venous blood was drawn; 3mL was collected on Ethylenediaminetetraacetic Acid (EDTA) containing tubes as whole blood, stored at -20°C till estimation of HbA1c% by commercially available HbA1c Ion Exchange Resin kit; provided by N.S Biotic Co., Alexandria-Egypt. The other 7mL was allowed to clot for 20 minutes at room temperature then centrifuged at 2000 rpm for 20 minutes. 3 mL of the supernatant was then transferred to RNase-free tubes and stored at −80°C till miR-155 analysis. The remaining 4mL was stored at -20°C till assay of serum glucose, creatinine.Ribonucleic acid (RNA) was isolated by using a Trizol-based miRNA isolation protocol (ThermoFisher scientific, Ambion TM, USA, TRIzol® Reagent, Cat. No: 15596-018). TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA, Cat. No: 4366596) and High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA, Cat. No: 438814) were used for reverse transcription. Serum levels of miR-155 were quantified by RT-qPCR using the ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Commercially available TaqMan primers, (Forward; CTAGCCTGCAGGTATTCAAATATTTCCACAGA and backward; ATCCGGCCGGCCTGAAGATGGTTATGAACATA), and probes, including 2 unlabeled PCR primers and 1 FAMTM dye-labeled TaqMan MGB probe, were used for all the targets (all from Applied Biosystems). For mRNA expression, the primer and probe set was deliberately designed across the intron-exon boundary so as not to detect probable genomic DNA. For RT-qPCR, 2.5 μL universal master mix, 0.25 μL primer and probe set, 0.33 μL cDNA, and 1.92 μL H2O were mixed to make a 5 μL reaction volume. Each sample was run in duplicate. RT-qPCR was performed at 50°C for 2 minutes and 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. β-Glucuronidase (GUSB, Applied Biosystems) and RNU48 (Applied Biosystems) were used as house-keeping genes to normalize the mRNA and miRNA expression, respectively [17]. Results were analyzed with Sequence Detection Software version 2.3 (Applied Biosystems). In order to calculate the differences of expression level for each target among samples, the ΔΔCT method for relative quantitation was used. Average expression level of normal blood samples from normal subjects was used as calibrator for serum expression and the expression level of targets was a ratio relative to that of the controls.Serum glucose was estimated by Max DiscoveryTM glucose assay kit manual Cat. No: 5611-01 (Bioo Scientific Corporation). Serum glycated albumin (GA) was estimated by MBS glycated albumin (GA) ELISA kit, Cat. No: MBS 160449. Serum NGAL was estimated by Human NGAL/LIPOCALIN-2Enzyme linkied immunosorbant assay (ELISA) kit supplied by Aviscera Bioscience, INC., 2348 Walsh Ave., Suit c, Santa Clara, CA95051, USA, Cat. No: skoo 233-02. Serum and urinary creatinine were estimated by alkaline picrate method. [18] Total albumin was estimated by bromocresol green albumin assay kit supplied by SIGMA-ALDRICH, 3050 Spruce st., Louis, MO, 63103 USA.A morning urine sample was collected from all participants, mixed well then centrifuged at 2000 rpm for 10 minutes. The supernatant was frozen at -20°C till the assay of urinary creatinine by alkaline picrate method [18] and urinary albumin by a solid phase ELISA kit, provided by DRG International INC., USA, Cat. No: EIA-236. The urinary albumin levels were adjusted for urinary creatinine [19].Collected data were reviewed and analyzed using the Statistic Package for Social Science Version 20 (SPSS 20.0) for windows. Data was summarized according to the randomized case-control design. Shapiro-wilk test was used to test the normality of the studied variables. T-test or Mann-Whitney test was used to compare 2 independent quantitative variables. Independent samples Kruskal-Wallis test oranalysis of one way variance (ANOVA) were used to compare more than two continuous quantitative data at a time. Chi square test was used to compare qualitative data. Studying the relationship between variables was done using Kendall’s-tau correlation and regression analysis. MedCalc Statistical software version (12.2.1.0) was used for determination of cut-off points, sensitivity, specificity and area under receiver operating characteristic (ROC) of serum miR-155, sNGAL regarding DN. The level of significance was taken at p-value of ≤ 0.05.
3. Results
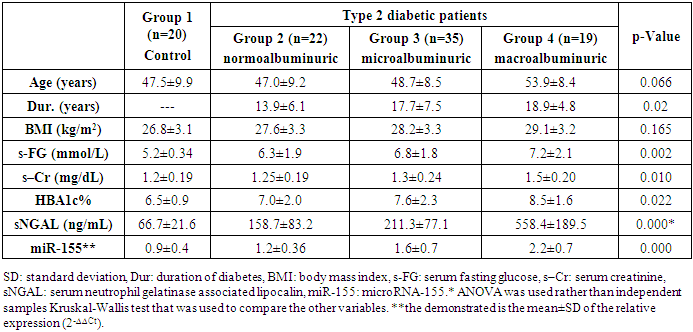
- The present study showed significant differences between the studied groups regarding serum fasting glucose, creatinine, NGAL, and miR-155 relative expression and HbA1c% (p= 0.002, 0.01, 0.000, 0.000, 0.022 respectively), with high values observed in group 4 followed, in order, by groups 3, 2 and 1. The same was observed regarding the duration of diabetes (p=0.02) (table 1).
|
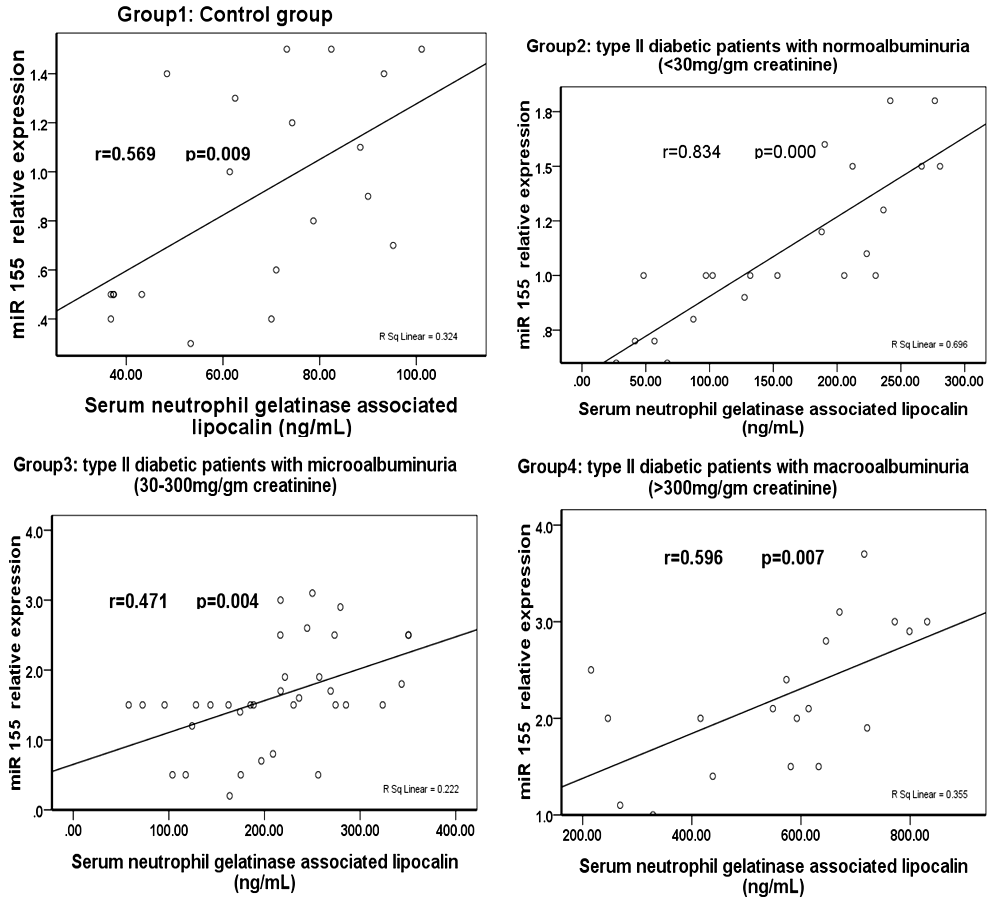 | Figure (1). Correlation between miR-155 relative expression and serum neutrophil gelatinase associated lipocalin in various studied groups |
4. Discussion
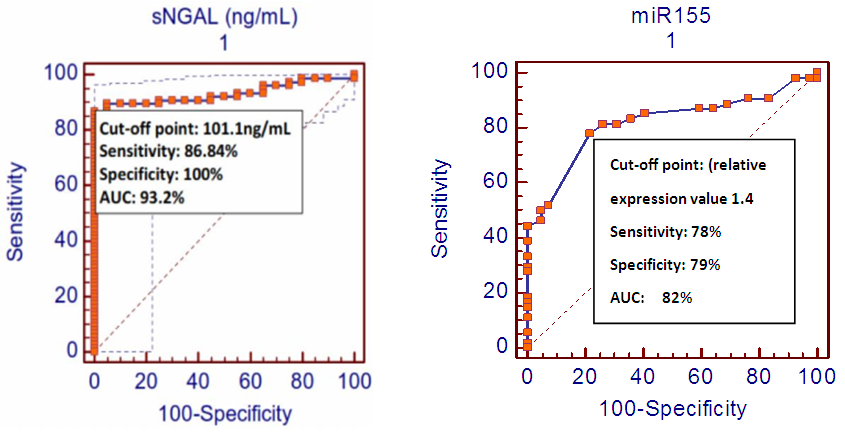
- Diabetes is a worldwide health problem with high risks of severe microvascular and macrovascular complications. Among these complications, DN may affect about 30% of patients with diabetes and commonly result in end stage renal disease. [28]Many clinical studies reported that early interventions are effective to slowdown the progression of the end stage renal disease once DN is established. [29, 30]Early prediction and diagnosis of DN is essential to allow tight glycemic control and so, to prevent the development of marked renal insufficiency [20]. Kamran, 2014 reported that detection of microalbuminuria predicts early the diabetic ischemic heart as well as diabetic kidney diseases [5]. He also reported that there wa a significant association between microalbuminuria, nephropathy, hypertension, and dyslipidemia and the development of ischemic heart disease. [5]Neutrophil gelatinase–associated lipocalin (NGAL) have been emerging as an early renal tubular biomarker that has a prognostic value in predicting of both acute and chronic nephropathies including DN [8, 10]. Also, the endogenously produced, short (21-25), noncoding RNAs i.e. MiRNAs, have important effects on key cellular functions by modulating gene expression [21]. There are many evidences that reported the vital role of miRNAs in regulating signaling pathways involved in the pathogenesis of DN. ThemiRNAs that induces DN were found to be upregulated in diabetic kidney while protective ones were found to be downregulated. [21]The present study is a comparative case-control that investigated the serum miRNA-155 (miR-155) levels in patients with diabetic nephropathy and healthy controls. Besides, it tested the correlation between serum miR-155 levels and sNGAL levels in these cases. The female percentages were found to be high in type 2 diabetic patients which is in accord with many previous studies. [22-24]The significantly high values of serum NGAL levels, in patients with DN, were in agreement with several previous studies [8, 9]. Also, the significantly high relative expression values of serum miR-155, in patients with DN was in accord to the finding reported by Huang his colleagues (2014). Theydescribed a pathogenic importance of miR-155 and miR-146a in DN and demonstrated that miR-155 and miR-146a were up-regulated in the DN patient and experimental animal models. They also found that miR-155 and miR-146a served as a mediator of the glucose-induced TNF-α/TGF-β1-NF-κB pathway (not only biomarkers) [27]. M oreover, the miR-155 and sNGAL values significantly increased in patients with normolbuminuria when compared to the control group i.e. before the appearance of microalbuminuria. This finding supports the earlier tubulointerstatial affection in DN even before glomerular involvement [26, 27].The significant correlation between miR-155 relative expression values and sNGAL levels in the present study may emphasize the pathogenic importance of miR-155 in DN reported by Huange et al. (2014) [25] and also add the miR-155 as biomarker for early diagnosis and prediction of DN.The cut-off point, sensitivity, specificity and AUC of the circulating miR-155 and sNGAL (ROC analysis), shown in figure (2), conclude a good diagnostic profile for both biomarkers in recognition of DN among all study participants even if they are normoalbuminuric.The main limitation of the present study is being of small sample size.In conclusion, the present pilot case control study has demonstrated that miR-155 relative expression values and sNGAL levels increase early in type 2 diabetic patients with DN even before the onset of microalbuminuria and they correlate with each other. This could improve the early diagnosis of DN and enhances its management protocol. It is recommended having further studies to emphasize the role of microRNAs in the pathogenesis and prediction of DN.
ABBREVIATIONS
- ANOVA: analysis of one way variance, AUC: area under receiver operating characteristic curve, DN: diabetic nephropathy, EDTA: Ethylenediaminetetraacetic Acid, ELISA: enzyme linked immunosorbant assay, F: females, HbA1c: glycosylated hemoglobin, KDa: kilodalton, M: males, NGAL: neutrophil gelatinase associated lipocalin, miRNA (microRNA or miR): microribonucleic acids, ROC: receiver operating characteristic curve, Rpm: round per minute, sNGAL: serum neutrophil gelatinase associated lipocalin, SPSS: statistical package for social science software, UA/CR: urinary albumin/creatinine.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML