-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2017; 6(1): 16-23
doi:10.5923/j.diabetes.20170601.03

Hypoglycemic and Anti-Inflammatory Effect of Gold Nanoparticles in Streptozotocin-Induced Type 1 Diabetes in Experimental Rats
Husam M. Edrees1, 2, Ayman Elbehiry2, 3, Yousif M. Elmosaad2
1Faculty of Medicine-Department of Physiology- Zagazig-University, Egypt
2College of Public Health and Health Informatics, Qassim University, Saudi Arabia
3Faculty of Veterinary Medicine, Department of Bacteriology, Mycology and Immunology, Sadat City University, Egypt
Correspondence to: Husam M. Edrees, Faculty of Medicine-Department of Physiology- Zagazig-University, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The high confidence that applying nanotechnology in medicine will convey major advances in the diagnosis and treatment of the disease prompted this technology to a variety of medical applications. The ability to use Gold nanoparticles for biological applications as anti-inflammatory has led to a great interest in studying and exploitation of their possible beneficial effects on biological systems and how to diminish its harmful effects. Therefore, this research aimed to detect the effect of gold nanoparticles (AuNPs) administration on glucose and insulin levels and its anti-inflammatory effect in rats. Design: A total number of 30 healthy adult male albino rats were used to study the effect of gold nanoparticles (10 nm size) through an intraperitoneal injection of 2.5 mg/kg b.w./day for 7 days on serum glucose, insulin, liver enzymes, creatinine, BUN and proinflammatory cytokines in Streptozotocin (STZ) induced diabetic rats. The rats were classified into 2 groups: Control group (Group I), STZ induced diabetic group (Group II) which is further subdivided into 2 groups, diabetic untreated control group (Group IIa) and diabetic AuNPs treated group (Group IIb). Results: This study results showed that all the parameters significantly increased in STZ induced diabetic group with a significant decrease in insulin level in comparison with the control group. On treatment with AuNPs, the mean values were significantly decreased for Serum glucose level and HOMA-IR (P<0.001, P<0.05 respectively), ALT, AST, ALP, Creatinine and BUN (P<0.001) with a significant decrease in TNF-α, IL-6 and CRP (P<0.001). However, all the parameters did not retain its normal level. Conclusion: Our results showed that AuNPs improved blood glucose level, liver enzymes and proinflammatory cytokines causing control of hyperglycemia, which therefore induce the possible role of AuNPs as a cost-effective therapeutic medication in the treatment of diabetes and its complications.
Keywords: Gold nanoparticles, Type 1 diabetes mellitus, TNF-α, IL-6
Cite this paper: Husam M. Edrees, Ayman Elbehiry, Yousif M. Elmosaad, Hypoglycemic and Anti-Inflammatory Effect of Gold Nanoparticles in Streptozotocin-Induced Type 1 Diabetes in Experimental Rats, International Journal of Diabetes Research, Vol. 6 No. 1, 2017, pp. 16-23. doi: 10.5923/j.diabetes.20170601.03.
Article Outline
1. Introduction
- Diabetes mellitus is a metabolic disease manifested with hyperglycemia and impaired glucose, lipids and protein metabolism [1]. This metabolic disorder is due to insulin secretion deficiency or a resistance to insulin action, or both [2]. Many metabolic abnormalities and chronic complications are generated from high glucose levels [3]. Type 1 diabetes (T1D) is a chronic autoimmune disease characterized by an absolute deficiency of insulin. Insulin deficiency is due to progressive damage of pancreatic islet beta cells due to autoimmune mechanism. Type 1 diabetes is supposed to be caused by unknown environmental factors in hereditarily vulnerable persons. The incidence of T1DM has been increased all over the world with an annual rate of about 3%. [4].Nanotechnology is used for controlling multiple medical processes with effective influence on medicine [5]. Colloidal gold nanoparticles (AuNPs) are utilized in many modern technological and medical applications because of its unique characteristic in interaction with visible light. Their optical and electronics characteristics gave the chance to be applied in electronic uses, for example, as sensory probes, Bioimaging, agents for medical therapy, drug delivery, medical applications and electronic instrumentalists. They are used in therapeutic agent carriage due to its large surface area/volume ratio, allowing their surface to be coated with numerous types of molecules including therapeutics and targeting agents [6]. The range of AuNPs use in recent medical studies is enormously extensive. In particular, it encompasses genomics, analysis of immunity, clinical chemistry and photothermolysis of microorganisms and cancer cells. Because of continuous increase in the variety of types of gold nanoparticle and their utilizations, toxicity and safety of these nanoparticles are gaining attention for well understand about the possible toxicological hazards of these novel substances [7]. The use of nanotechnology in medicine as a therapy for diabetes mellitus has been applied lately [8] through developing many antidiabetic agents for controlling diabetes using in vitro and in vivo research methods, however, some of these agents are restricted due to their pharmacokinetic possessions [9]. Generally, some metal nanoparticles, especially, AuNPs are utilized for the management of many medical problems because of their exclusive optical, biochemical and biological characteristics [10]. Indeed, preparation of AuNPs by biosynthesis procedure has deep antidiabetic potential [11]. It has been widely investigated for controlling of the blood glucose levels in hyperglycemia and diabetes as it contains numerous active compounds [12].The cytokine TNF-α is elaborated in autoimmune diseases causing damage of beta-cell followed by chronic hyperglycemia revealing a complex relationship with type 1 diabetes. Hyperglycemia and TNF-α impaired the insulin signaling. Resistance to Insulin related to obesity has an important role in atherosclerosis in vascular lesions, this was found to be caused by TNF-α [13]. TNF-α shows an important role both in complicated and non-complicated type 1diabetes mellitus. Studies performed on non-obese diabetic mice (NOD) showed that the administration of exogenous TNF-α controlled the development of diabetes mellitus in case of low production of endogenous TNF-α. High level of TNF-α, IL-6 and CRP levels were assessed in the newly diagnosed type 1 DM in children [14].AuNPs have anti-inflammatory properties through their capability to hinder expression of NF-kappa B and consequent inflammatory reactions [15-17]. Many researchers have studied the anti-inflammatory effect of various nanoparticles and the releasing of pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin (IL)-6 have been studied previously. The cytotoxic effects of gold nanoparticles were evaluated in macrophages and the expression of the inflammatory cytokines IL-6, IL-10 and TNF-α were quantified. It was found that NPs are cytotoxic to macrophages and are able to elicit an inflammatory response [18].The AuNPs with a diameter 10 and 50 nm have been studied in the experimental rat liver by Khan et al. [19] to express the effect of interleukin-1 beta (IL-1b), IL-6 and TNF-α. They found that there is a significant increase in cytokine gene expression at 10 nm. Nevertheless, AuNPs of 50 nm in size created a powerful stimulation of pro-inflammatory cytokines. These findings point to the potential biocompatibility of medium-sized AuNPs. Former in vitro study on murine macrophages indicated that 60 nm AuNPs have no cytotoxic effect and don’t impede the pro-inflammatory reactions of IL-6 and TNF-α [20]. Moreover, it was noticed that silver and gold nanoparticles can enter the cells, but that only the AuNPs particularly those with smaller diameter up regulate the expression of pro-inflammatory genes (IL-1, IL-6, TNF-α) [21]. They doubt that part of AuNPs with negative charge may adsorb serum protein and enter cells by endocytosis, leading to high level of cytotoxicity and immunological reaction of AuNPs than silver nanoparticles (AgNPs). The toxicological behavior of gold nanoparticles is not clearly understood. The cytotoxicity of gold nanoparticles has been studied in human cells and the results have shown that AuNPs are nontoxic up to 250 nm. Over-all, most works have excluded the toxicity of AuNPs (4–18 nm). Functionalized gold particles with a size of 13–20 nm do not cause acute adverse effects [19]. As the increased utilization of nanoparticles for controlling multiple medical processes and use of nanotechnology in medicine as a therapy for diabetes mellitus, the present study was designed to detect the role of AuNPs administration on glucose and insulin levels and clarifying its anti-inflammatory effect in rats.
2. Material and Methods
2.1. Experimental Animal
- This research was run out on 30 adult male albino rats 8 – 10 weeks old weighing 90-110 gram received from the faculty of veterinary medicine animal house, University of Zagazig. All the animals were preserved in steel wire cages (5/cage) in the research laboratory in physiology department, faculty of medicine, Zagazig University under sterile environments. Rats had normal mixed commercial rat laboratory diet with free access to water and chow supplied in clean dishes. Animals are maintained at temperature of room and were kept on a twelve hours of light and dark cycle. To avoid effect of different food elements on the experiments, all the rats were fed similar type of food [22]. Before the experimentations were going on by two weeks, accommodation of the rats was done to laboratory conditions. All procedures in this study were approved by the ethical committee of the Faculty of Medicine of Zagazig University. Rats were grouped into:Group I: (Control group – n. of rats = 20): rats were used as a control group; they received normal diet.Group II: (Streptozotocin (STZ) induced diabetic group - n. of rats = 10): Induction of experimental type 1 diabetes was done by intraperitoneal injection of 65 mg/kg of body weight of fresh streptozotocin solution at PH 4.5 and dissolved in 0.2 mmol/L sodium citrate [23, 24]. All animals were fasted overnight to avoid incidental puncture of the intestines, which may decrease the achievement rate for induction of diabetes. Confirmation of induced Diabetes was done after three days through measuring blood level of glucose in the blood sampled which is obtained from the tail vein) with the One Touch Ultra Glucometer. Diabetic rats (blood glucose level more than 8.88 mmol/L or 160 mg/dl) were selected for experiments [25, 26]. Then, diabetic rats were subdivided into two groups:Group IIa: Diabetic untreated control group (DC - n = 10): In which diabetes-induced rats served as the diabetic control group.Group IIb: Diabetic AuNPs treated group (DT - n = 10): In which diabetes-induced rats treated with gold nanoparticles (10 nm). Intraperitoneal injection of AuNPs started on the day number 5 after Streptozotocin injection. This is the day number one of treatment. AuNPs nanoparticles were injected at a dosage of 2.5mg/kg b.w./day to each rat in group IIb through intraperitoneal injection for 7 days [27, 28]. The control group received only citrate buffer [28].
2.2. Drugs and Chemicals
- 2.2.1. Gold Nanoparticles: AuNPs purchased from PlasmaChem GmbH, Rudower Chaussee 29, D-12489 Berlin, Germany. AuNPs colloidal solution (Cat. No: PLAu-S10-0.05 mg, 10 nm) were used in the present study. 2.2.2. Glucose (anhydrous): ADWIC Laboratory Chemicals, Egypt.2.2.3. Streptozotocin, [N-(Methylnitrosocarbamoyl)- α-D-glucosamine]: (SIGMA-ALDRICH Co.-USA).2.2.4. Rat Tumor Necrosis Factor alpha (TNF-α): ELISA Kit (Ray-Biotech, Inc. U. S. A.).2.2.5. Interleukin-6 (IL-6): ELISA Kit (Ray-Biotech, Inc. U. S. A).2.2.6. High-sensitive C-reactive protein (CRP): SIGMA-ALDRICH Co.-USA.
2.3. Laboratory Analysis
- 2.3.1. Measurement of blood glucose level: Using glucose oxidase-peroxidase (GOD-POD) glucose Measurement Kits (Biotechnology, Egypt) according to Trinder [29].2.3.2. Measurement of serum insulin level: Using the method described by Starr et al. [30], serum insulin level was assessed with solid phase enzyme amplified sensitivity immunoassay using KAP1251-INSEASIA (Enzyme Amplified Sensitivity Immunoassay) Kits (BioSource Europe S.A., Belgium).2.3.3. Determination of insulin resistance (HOMA-IR): It was evaluated by homeostasis model assessment (where HOMA-IR= (fasting insulin (μU/ml) x fasting plasma glucose (mmol/L) /22.5.2.3.4. Measurement of Serum Liver enzymes: According to Murray [31] and Young [32], the level of alanine transaminase (ALT) were measured using lactate dehyrogenase (LDH) -NADH kinetic ultra-violet reaction. The level of aspartate transaminase (AST) was assessed using multi dehyrogenase (MDH) -NADH kinetic ultra - violet reaction, in accordance with the procedure described by Murray [31] and Tietz et al. [33]. Using p-Nitrophenylphosphate kinetic reaction described by Wenger et al. [34] and Tietz et al. [33], the level of alkaline phosphatase (ALP) was determined.2.3.5. Measurement of Serum Creatinine Level: Using the method described by Murray et al. [31], serum creatinine level was assessed with creatinine kits supplied by SPINREACT, S.A.U. Ctra.Santa Coloma, 7E-17176 SANT ESTEVE DE BAS (GI) SPAIN.2.3.6. Measurement of Serum Blood Urea Nitrogen (BUN) Level: Using the method described by Murray et al. [31] using urea kits supplied by SPINREACT, S.A.U. Ctra.Santa Coloma, 7E-17176 SANT ESTEVE DE BAS (GI) SPAIN.2.3.7. Assessment of Serum Tumor Necrosis Factor alpha (TNF-α): assessment was performed by rat enzyme linked immuno-sorbent assay (ELISA) kits produced by Ray-Biotech, Inc. U. S. A.2.3.8. Assessment of Serum Interleukin-6 (IL-6): Analysis was performed by rat enzyme linked immuno-sorbent assay (ELISA) kits produced by Ray-Biotech, Inc. U. S. A.2.3.9. Assessment of Serum C-reactive protein (CRP): Analysis was performed by rat enzyme linked immuno-sorbent assay (ELISA) kits produced by Ray-Biotech, Inc. U. S. A.
2.4. Statistical Analysis
- The findings of this study are presented as mean ± standard error of the mean. We accomplished statistical analysis with the Statistical Package for the Social Sciences (SPSS), version 20.0. Repeated evaluations with analysis of variance (ANOVA) were applied. P value <0.05 was considered to be statistically significant.
3. Results
3.1. Serum Glucose, Serum Insulin and HOMA-IR
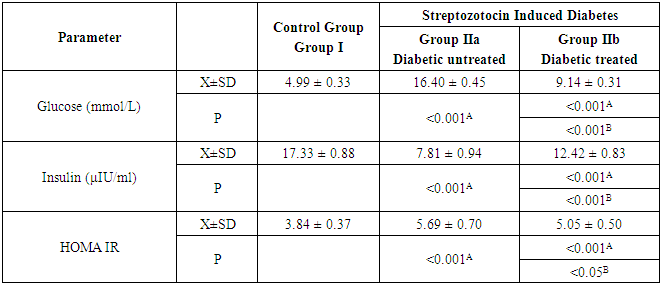
- As shown in Table 1 and Figure 1, the mean values of serum glucose level and HOMA-IR in diabetic untreated control group (group IIa) were 16.40 mmol/L ± 0.45 and 5.69 ± 0.7 respectively. It was noticed that these values were significantly higher (P<0.001 & P<0.001) when compared with that of control group (group I). In contrast, the mean value of serum insulin level (7.81µ IU/ml ± 0.94) was decreased significantly (P<0.001). When we compared diabetic AuNPs treated group (group IIb) with that of group IIa, the mean values were significantly decreased for serum glucose level (P<0.001) and HOMA-IR (P<0.05) and significantly increased for serum insulin level (P<0.001). Nevertheless, after comparing group IIb with that of group I, it was noticed that the mean values were significantly increased for serum glucose level (P<0.001) and HOMA-IR (P<0.001) and significantly decreased for serum insulin level (P<0.001).
|
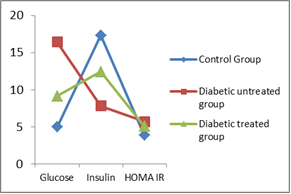 | Figure 1. Serum glucose (mmol/L), insulin (μIU/ml), HOMA index of insulin resistance |
3.2. Serum Liver Enzymes (ALT, AST and ALP), Serum Creatinine Level and Serum Blood Urea Nitrogen
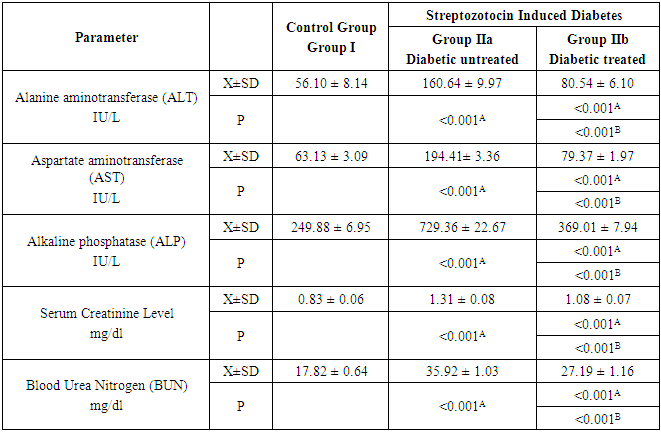
- In group IIa, the mean values of ALT, AST, ALP, serum creatinine level and serum blood urea nitrogen were 160.64 ± 9.97 IU/L, 194.41 ± 3.36 IU/L, 729.36 ± 22.67 IU/L, 1.31 ± 0.08 mg/dl and 35.92 ± 1.03 mg/dl respectively. While in group IIb, these values were 80.54 ± 6.1, 79.37 ± 1.97, 369.01 ± 7.94, 1.08 ± 0.07 and 27.19 ± 1.16 respectively. After the comparison between group IIa and group IIb with that of group I, it was noticed that the mean values were increased significantly (P<0.001) for all measured parameters. Nevertheless, on comparing group IIb with that of group IIa, the mean values were decreased significantly (P<0.001) for all measured parameters (Table 2 and Figure 2a & b).
|
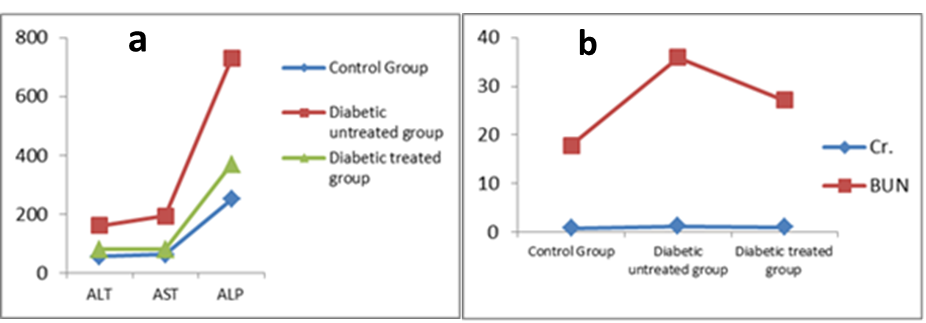 | Figure 2. a) Serum ALT, AST and ALP (IU/L), b) Serum Creatinine and BUN (mg/dl) |
3.3. Serum Cytokines: TNF-α, IL-6 and CRP
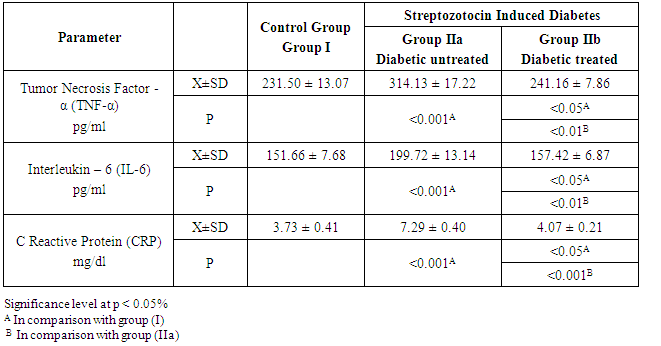
- In group IIa, the mean values of TNF-α, IL-6 and CRP were 314.13 ± 17.22 pg/ml, 199.72 ± 13.14 pg/ml and 7.29 ± 0.40 mg/dl, respectively. While in group IIb, these values were 241.16 ± 7.86 pg/ml, 157.42 ± 6.87 pg/ml and 4.07 ± 0.21 mg/dl, respectively. From these results, it was found that the mean values in group IIa were significantly higher for all measured parameters (P<0.001). On comparing group IIb with that of group IIa, the mean values were significantly lower for TNF-α, IL-6 and CRP (P<0.01, P<0.01 & P<0.001 respectively). The mean values of group IIb were found to be significantly high (P<0.05) for all measured parameters when compared with that of group I as shown in Table 3 and Figure 3.
|
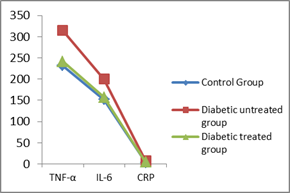 | Figure 3. Serum TNF-α (pg/ml), IL-6 (pg/ml) and CRP (mg/dl) |
4. Discussion
- Using of AuNPs as anti-hyperglycemic and anti-inflammatory agents have received a great attention in treating diabetes mellitus [35]. The main purpose of the present work was to validate the anti-hyperglycemic effect of AuNPs of size 10 nm. This was established with the major decrease of blood sugar levels that reinforces the anti-diabetic effect of AuNPs in experimental rats.Our study indicated that the mean glucose level was significantly augmented, while the mean insulin level was significantly decreased in the diabetic non-treated group of rats compared with the control group. After intra-peritoneal injection of AuNPs at a dosage of 2.5 mg/kg.b.wt for 7 days, the blood glucose level and HOMA IR were significantly decreased, whereas; the serum insulin level was significantly increased compared with the diabetic non-treated group. Conversely, the blood sugar level was still away from the control level. These findings are in accordance with the former study descriped by Barath Mani Kanth et al. [36] who stated that the blood glucose level was decreased significantly after treatment of AuNPs compared with the diabetic control group. In contrast, another study made by Selim et al. [28] who indicated that there was no difference between the blood glucose level and control level. Furthermore, Karthick et al. [37] stated that treatment of experimental rats with AuNPs led to significant reduction of the blood glucose level and considerable increase of serum insulin level. In contrast to our study, they found that the diabetic rats treated with AuNPs significantly (p<0.001) brought the blood glucose and serum insulin levels to the normal level which is considered an evidence of the hypoglycemic effect of the AuNPs.The liver enzymes AST (aspartate transaminase), ALT (alanine transaminase) and ALP (alkaline phosphatase) are metabolic enzymes, which have the ability to escape into the blood stream as a result of liver damage due to hyperglycemic conditions. The findings of the current study demonstrated that in STZ-induced type 1 diabetic rats, the mean of serum levels of liver enzymes ALT, AST & ALP, blood urea nitrogen and creatinine were significantly increased (p<0.001) in comparison with the control group. Treatment of experimental rats with AuNPs at a dosage of 2.5 mg/kg.b.wt for 7 days significantly decreased the level of liver enzymes (p < 0.001) confirming their capability to protect the liver from damage, while the significant reduction of blood urea nitrogen and creatinine levels (p < 0.001) indicating the curative effect of AuNPs on renal function. Nevertheless, the mean is still higher than that of control group (p<0.001). Our findings are similar to the results obtained by Barath Mani Kanth et al. [36] who exhibited the potential effect of AuNPs to reduce the liver enzymes, blood urea nitrogen and creatinine close to the normal levels relative to controls. In addition, they found an important decrease in fatty cells, normal central vein with no ground glass nuclei with, stating the restorative effect of AuNPs over the organ damage in diabetic induced mice treated with AuNPs.Furthermore, Selim et al. [28] studied the effect of the AuNPs on the various metabolic enzymes level (measuring the effective functioning of the liver) and creatine and uric acid levels as an indicator of renal damage in STZ induced diabetic rats. They illustrated that there is a significant decrease of creatine and uric acid levels compared with the control group. Conversely, to our findings, Doudi and Setorki [38] and Mak and Moussa [39] demonstrated that creatine and urea levels were increased (non-significant) after treatment with 10 nm and 50 nm nanoparticles compared to the control group. These non-significant changes can be due to the high clearance of AuNPs through the kidney or the short period of the treatment.TNF is recognized to show double role in autoimmune diabetes based on exact timings where the autoimmune process in type 1 diabetes is determined by inflammatory agents [40]. Increased serum levels of pro-inflammatory cytokines such as IL-6 and TNF- α represent the state of insulin resistance. AuNPs have received great attention as an anti-inflammatory mediators. In the current study, we found that the mean serum level of TNF-α, IL-6 and CRP, augmented significantly in STZ-induced type 1 diabetic rats. These results confirmed the harmful role of proinflammatory cytokines in the pathogenesis of type 1 diabetes. After treatment of experimental rats with AuNPs at a dosage of 2.5 mg/kg.b.wt for 7 days, the level of these cytokines was significantly decreased compared with that of group IIa. The mean values for all measured parameters of group IIb were found to be decreased close to that of group I; but it still significantly higher (P<0.05) for all measured parameters. These results indicate the role of gold nanoparticles in reducing the levels of pro-inflammatory cytokines and suppressing the inflammation and hence improving the pathophysiology of type 1 diabetes. Our findings are similar to that described by Karthick et al. [37] who investigated the effect of AuNPs on TNF-α, IL-6 and CRP in serum of control and treated rats. They revealed that after treatment with AuNPs, the serum levels of TNF-, IL-6 and CRP were decreased significantly to the normal levels as compared with that of the diabetic group, indicating the effect of AuNPs on suppressing the inflammation.
5. Conclusions
- The results of the current research, propose that treatment with AuNPs at a dose of 2.5 mg/kg.b.wt for 7 days in Streptozotocin Induced Diabetes improved blood glucose level, insulin resistance, insulin sensitivity, liver and kidney performance. Using of inflammatory markers TNF-, IL-6 and CRP, we conclude that AuNPs also have an anti-inflammatory effect on diabetic rats. Based on these results, we suggested that colloidal AuNPs could be utilized in applying nanotechnology in medicine for treatment of diabetes. We conclude that the low costs of production and relative availability of modification of gold nanoparticles make it practicable for future biomedical applications. Additional researches are needed to clarify the toxicity of AuNPs on different tissues and organs.
ACKNOWLEDGMENTS
- The authors would like to express their appreciation to the supportive team of technical specialists in the Physiology Department, Zagazig University for their valuable help in the study work and laboratory analysis. This study received no special funding from any agency in the public or private sectors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML