-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2016; 5(6): 123-128
doi:10.5923/j.diabetes.20160506.01

Insulin/Enzyme Calculator “Gourmet” for Type 0 Diabetic Patients: Why, What for, and How?
Igor Peshko
Wilfrid Laurier University, Physics and Computer Science Dep., Waterloo, Canada
Correspondence to: Igor Peshko, Wilfrid Laurier University, Physics and Computer Science Dep., Waterloo, Canada.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This paper is an introductive chapter in the Insulin/Enzyme Calculator series. The prototype of “Gourmet” was developed to assist diabetic patients in controlling blood sugar after a total pancreatectomy or Whipple procedure. Taking into consideration the causes and symptoms of different types of diabetes, it is proposed to classify the post-surgical cases as type 0 diabetes. Since such patients have a significantly modified digestive system, new approaches in blood sugar analysis and treatment should be developed. This paper reviews existing mathematical models of diabetes and indicates how “Gourmet” compares to available simulators. It specifies the main problems with precise insulin dose estimation and describes the basic operational principles of the proposed computer program. The processes that must be taken into account are as follows: dynamical blood sugar profile related to digestion of carbohydrates of different types, sugar contribution by the liver, fat/proteins long- and short-term contributions into blood sugar level, influence of the enzymes and bicarbonate on blood sugar behavior, difference in carbohydrates content and their relative portion in cooked and uncooked meals.
Keywords: Insulin/Enzyme Calculator, Type 0 Diabetes, Mathematical Models of Diabetes
Cite this paper: Igor Peshko, Insulin/Enzyme Calculator “Gourmet” for Type 0 Diabetic Patients: Why, What for, and How?, International Journal of Diabetes Research, Vol. 5 No. 6, 2016, pp. 123-128. doi: 10.5923/j.diabetes.20160506.01.
Article Outline
1. Introduction
- Next day after surgery, Dr. Jayaraman visited his patient, Dr. Peshko. He said: “Igor, you do not belong to the human race anymore. Listen to your body and think about what can be done to help yourself.”The author of this paper, Dr. Peshko was diagnosed with “giant cells” pancreatic cancer in August 2014. He underwent surgery in October 2014 (Whipple procedure), followed by a course of preventive chemotherapy. Being unable to manage extremely unstable blood sugar behavior by traditional methods, Dr. Peshko developed his own techniques that significantly improved his blood sugar control. Most of the traditional insulin dose estimation instructions are focused on the correction of residual sugar values [1]. However, simple notion “insulin vs. sugar” does not satisfy real-life requirements. Patients eat varied types and amounts of food, experience different physical exertion, and follow different meal/injection time schedules. Moreover, each patient has an individual intensity of different organ secretions. The inability to take into account all these variables can easily result in uncontrollable blood sugar fluctuations that reduce quality of life and typically have long-term health complications. It becomes evident that there is a need for a new approach that is capable to simultaneously consider multiple factors driving blood sugar behavior. In any area of human activity, there is a gap between research and applications. Practical medical guides that are used in the hospitals today typically contain instructions that are 10-15 years old. The results of new research should be discussed and verified multiple times, officially approved, prepared as textbooks, and, finally, medical staff should be educated and trained. The development of appropriate software with a user-friendly interface can significantly accelerate the process of knowledge transfer, which can be shortened from tens of years to just a few months or even weeks.This series of papers is focused on practical and immediate application. We tried to use plain language and fill the gap between regular patients and medical research.
2. Approach
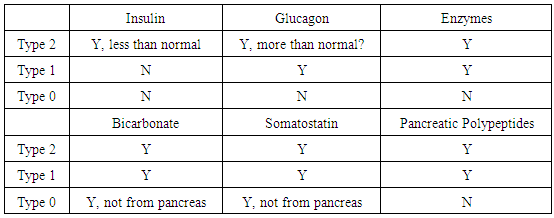
- In order to develop an appropriate treatment technology, the class of diseases and problems should be specified first. In Dr. Peshko’s case, the Whipple procedure was performed: three organs (pancreas, gall bladder, and spleen) were removed completely and two (stomach and duodenum) partially. Currently, diabetes management guides classify such patients as type 1 as they become completely insulin dependent. However, classic type 1 and post-surgical cases are not of the same nature. The cause of type 1 diabetes is a disorder of the immune system that attacks insulin-producing cells [2, 3]. In the post-surgical case the nervous, blood, lymphatic, and gall channels are interrupted or modified; the normal system of food digestion is practically destroyed. In the case of pancreas absence, not only insulin is not produced, but enzymes, glucagon, somatostatin, pancreatic polypeptides, and other important secretions are missing; their derivatives are lost as well. Traditionally, the management of post total pancreatectomy diabetes was regarded as challenging. It requires low, precise doses of insulin since it is characterized by frequent and severe hypoglycemic events [4]. Due to difficulties of carbohydrate and enzyme amount estimation, and uncontrollable liver activity, the issue of hyperglycemic events should be addressed as well. The American Diabetic Association classifies pancreatogenic secondary diabetes associated with the disease of the pancreas, including cases after pancreatectomy, as type 3c [5]. Multiple publications over the past years compare the long-term health state of the patients that suffer from diabetes of different types (e.g. [6, 7]) and conclude that pancreatectomy cases are similar to type 1 and can be managed in a similar way. Reference [7] analyses the differences in HbA1c values for post-pancreatectomy and classic type 1 diabetes cases. It was found that HbA1c values averaged within the two groups of patients are similar. Since HbA1c characterizes the average blood sugar level during the period of approximately 6-12 weeks, it provides no information about blood sugar stability. The repeatable sugar variations in the range of 10±7 mmol/L or 10±1mmol/L can result in the same HbA1c level, but not in the same patient conditions and long-term complications. Also, patients with identical levels of blood sugar can have a difference around 1% of HbA1c, which corresponds to 1.6 mmol/L averaged blood sugar concentration [8, 9]. Since the post-Whipple patients have no the spleen, the old red blood cells are not recycled and platelets and white blood cells are not stored. In this situation it is not obvious what is a real portion and HbA1c in such unfiltered blood? Recently the wide group of diseases and organs dysfunctions was classified as type 3 diabetes [10, 11]. It is associated with brain diseases (stroke, dementia, Alzheimer’s and neurodegenerative diseases, like Parkinson’s one). It should be emphasized that post-Whipple patients can typically have a number of problems that are not directly related to diabetes but introduce additional obstacles during diabetes treatment. These are high stomach acid, very low hemoglobin level (especially after a course of chemotherapy), high blood pressure, stomach dysfunction (due to dissection and diminished volume), and unacceptability of drugs and foods (due to strong nausea and sensitivity) during the first three months after the surgery. Due to absence of spleen the post-Whipple patients are unprotected against pneumonia and meningitis.As far as type 0 diabetes is considered, the special protocols for insulin application should be developed for post-surgery patients. Moreover, during the process of recovery, the state of the patient can typically change dramatically. The recommendations provided immediately after the surgery should be modified to match changing patient conditions. Finally, as it was for Dr. Peshko, the patient may not even experience typical symptoms of type 1 diabetes: increased thirst, appetite, and so on. In the absence of dozens of biochemical substances, with broken feedback mechanisms and modified microbiological background, we believe that insulin dependence is not a sufficient factor to assign the post-surgical patients to type 1 diabetes group. In lay terms, it is similar as to claiming that a car and a motorboat can be considered belonging to the same group because both of them are “gas-dependent”. In order to differentiate type 1 and post-surgical diabetes, we propose to classify the latter as type 0. Table 1 illustrates the difference in secretion availability for different types of diabetes, considering a few basic secretions (Y – available, N – not available, ? – under discussion). It is evident that the maximal number of absent secretions is associated with type 0 diabetes.
|
3. Mathematical Modeling of Diabetes
- A critical review of different diabetes models and data used in diabetology can be found in [12]. Here we briefly review several systems that may help place our work in context. Since the 1970’s, the mathematical methods of diabetes analysis were being developed very intensively. Dr. R.N. Bergman and co-workers initiated mathematical modeling of the insulin action [13]. They studied blood sugar dynamics within the framework of the “minimal model” (MM). Briefly, the technique uses a set of differential equations, the solution of which should fit the experimental curve by variation of unknown coefficients. In such a way, the blood sugar or insulin dynamical behavior could be described mathematically. However, being a mathematic technology MM has its own problems: stability of solutions, multiple or not real solutions, etc. It should be emphasized that in the MM, the formal description of the insulin/glucose dynamics does not explain real physiological processes (like liver sugar production or insulin/glucagon-liver interaction) or properties of consumed substances (foods and medications). It considers dynamics of the “final players”: insulin and blood sugar. In fact, MM has not been developed specifically for diabetes; this is a method capable to fit a set of discrete data to an analog curve that may describe several interdependent processes, such as insulin and blood sugar dynamics. In any case, the MM served as a trigger for thousands of researchers who aimed modeling and describing the diabetes. The next significant step was an approach called Archimedes [14, 15]. It includes four main kinds of mathematical models in health care: 1) Biological modeling, 2) Clinical medicine, 3) Operations research, and 4) Economic/system resources. Archimedes is also written in differential equations, with a total number of variables of several hundred! Different levels of detail may be considered [14]. However, this powerful instrument is not suitable for personal use at home; it is more focused on trends of disease, economical efficiency of health care processes rather than on solving the problem of a regular patient, such as each time precise medication doses estimation. The concept postulates some effects without analysis of the reasons of phenomena. Let us consider an example [14]: “In people who develop type 2 diabetes, the simulated liver cells develop a resistance to the effects of insulin. This causes the simulated liver to produce too much glucose. In response, the simulated β-cells produce more insulin. Over time, this compensatory mechanism begins to fail through a combination of decreased insulin production (e.g., “β-cell fatigue”) and increasing resistance to insulin by the liver. ... “. This paragraph discusses the concept of “resistance”; mathematically, it can be expressed as some diminishing factor (coefficient) in the equations, and result in good fitting of the experimental and simulated blood sugar dependencies; however, it is unclear how that can be translated into practice, i.e., what specific treatment action should be taken. The review devoted to analysis of the “resistance” reasons [17] states: “Insulin resistance in most cases is believed to be manifest at the cellular level via post-receptor defects in insulin signaling. Despite promising findings in experimental animals with respect to a range of insulin signaling defects, their relevance to human insulin resistance is presently unclear…“ The author quoted about 7 processes that can result in insulin resistance. Hence, to be helpful for a specific patient, Archimedes should be detailed down to particular physiological processes, responsible for the “resistance”. Finally, the model does not expect absence of several organs, such as the case after Whipple procedure. A zone of special attention is a set of programs focused on blood sugar prediction. As an example, [18] can be considered. The authors develop a new data-driven approach for modeling glucose dynamics of type 1 diabetic patients, accounting for effects of insulin and meal intake by the means of differential equations with time-varying coefficients. However, it should be noted again that manipulation with coefficients of differential equations can result in practically ideal fit of discrete data and simulated analog graph for some specific group of patients and some certain conditions. However, any new peculiarity of patient physiology (like in Dr. Peshko example) will result in bad fitting of the data because real background processes were not taken into account. The mathematical models typically use some phenomenological parameters to explain sugar behavior profile, like discussed above “insulin resistance”, or “insulin sensitivity”, etc. In our experience, such parameters as carbohydrate/insulin sensitivity depend on the time of day, the previous day’s meal content, physical and mental activity before the meal, amount of taken enzymes, body site for insulin injection, and so on. Hence, when the medical guides inform that carbohydrate/insulin sensitivity can vary from 5 g to 30 g per insulin unit for different patients, (e.g., [3]), it should be specified at what conditions those measurements were acquired. If measurements were done before lunch or after dinner, for sleeping patients or making physical exercises the numbers will be different for each “unique” patient. We would like to emphasize that at this point the processes related to insulin resistance are not included in the Calculator algorithm, as more research has to be done. Moreover, in case of pancreas absence the role of secretions that are not actual are not discussed (like in [19].The proposed Gourmet Calculator is simpler in use than the approaches discussed above. Calculator Version 1 has been developed as an “emergency” prototype, and as such, it is focused on the following problem: how to achieve a blood sugar level that is maximally stable and close to clinically acceptable. This was done by monitoring and controlling food content and cooking technologies (starch/sugars ratio, carbohydrate and water partial amounts), artificial enzymes (sugar generation rate), preprandial sugar level (history of sugar accumulation), and daily profile of liver activity (glycogen/glucose both-side conversion). Version 1 does not consider fat/proteins contribution into blood sugar dynamics, digestion time and sugar generation rate profile of different kinds of carbohydrates.
4. Results: Calculator Concept
- The main question of Calculator development is what principles should be used as the basic concept. Traditionally, there is a notion in medical community that due to the uniqueness of each patient, it is impossible to describe, by formulas, the body’s internal processes and to develop treatments based on data of one patient. Instead, the research is focused on accumulation of data from large number of different patients and on development of the mathematical methods that can approximate these data by a relatively simple dependence. While this approach may be good in describing the results of action of large number of interconnected processes, it is unclear how it could benefit a particular patient on a daily basis. After many years of successful development of probabilistic models of physical processes based on limited observations, Dr. Peshko's opinion is that even small data, reinforced by understanding of the underlying processes, can result in the design of the models applicable to multiple patients. While it is certainly beneficial to test Calculator concepts on multiple patients, this may be currently challenging due to a number of objective reasons. First of all, the author has no access to the data of other patients. Secondly, to better understand the investigated processes it is required a scrupulous recording, day by day, of the following parameters: time of meals, amount and types of carbohydrates, proteins, and fats in each food component, values of consumed enzymes, injected insulin doses, blood pressure and heart beat rate, as well as hemoglobin, HgA1c, and liver and kidney function parameters monthly. Dr. Peshko recorded these parameters for a year and a half, and at the same time has no knowledge of anyone else who has collected similar data. Dr. Peshko would like to emphasize again that “one case research” can provide valuable information (as compared to statistical descriptive medicine) if the research is targeted towards consideration of basic processes running in a single patient. If a detailed picture is clear, one can simulate and compare the results for other patients by variation of parameters of separate processes. In this case, we have a chance to understand what is wrong with each patient and develop an individual treatment strategy.We believe that this research presents a very interesting opportunity: post-pancreatectomy patients can be considered as a model system for simulation of different patients. Taking different doses of insulin, enzymes, bicarbonate pills, and so on, it is possible to not only study the reactions of type 0 patients, but also to imitate type 1 or 2 patients, modeling for whom may be more complicated as the available pancreas generates perhaps small but unknown and unrepeatable amounts of these secretions. In case of type 0 patients, we can experimentally examine the blood sugar-insulin dependency when pancreas does not participate in driving the blood sugar level. In some sense, most type 0 patients are pretty similar since they do not have a number of principle secretions, and consequently, no variety of these secretions that shapes the uniqueness of patients. Another interesting possibility lies in interpretation of the available data. For example, the author of this paper had different weight, physical activity, blood parameters, blood pressure behavior, etc. during each month of the recovery period. Hence, his long-term data can be split into shorter chunks that can be viewed as the short-term data of different patients.To summarize, we plan to iteratively develop the Calculator by gradually adding the following features working on the following concepts:1) Take sugar generation from carbohydrates and liver contribution into account; maximally precisely estimate total amount of taken carbohydrates; consider different cooking technologies affecting relative portion of starches and light sugars in food and portion of water in cooked meals; follow sugar accumulation process to correct current insulin dose. This version is practically ready and will be described in next papers in detail.2) Study digestion dynamics of different types of carbohydrates (starches and sugars) and find protocols of insulin injections helping to avoid hypoglycemia and hyperglycemia events. The mathematical modeling and preliminary experiments demonstrate an exception role of the dynamical processes of sugar generation and assimilation: taking the same amount of carbohydrates and injecting the same corresponding dose of insulin it is possible to get high, low, or normal sugar level, depending on content and order of consumption of carbohydrates in a meal.3) Develop a new data acquisition and processing methods capable to answer such questions: What is an optimal interval between blood sugar measurements that provides maximum information at minimal number of measurements? How to extract the correct information from data achieved with a low-accurate blood sugar meter? What is the best data interpolation method for investigation of blood sugar dynamics, insulin operational rate profile, or food digestion related processes?4) Explore proteins/fats metabolism and estimate long-term sugar contribution from these sources. Develop long-term insulin injection protocols (tens of hours) taking into account fat/protein consumption. 5) Describe the processes of sugar consumption and connect them to patient physical activity and environmental conditions (temperature and atmospheric gas content). 6) Create database with information about natural enzymes contained in plant foods and develop Optimal Menu Generator for specific patient and conditions. 7) In addition, we are developing the software package (Patient Endocrine Portrait) capable to predict blood sugar level 4 hours after the insulin injection. The proposed method is based on previous blood sugar statistics of the patient and helps to avoid invasive blood sugar measurements over, at least, an 8-hour period. This version is ready and will be demonstrated soon. It should be understood that Calculator is not a competitor to insulin pumping systems (like Dexcom [20]). The pump systems analyze available sugar concentration and aim to stabilize it. The Gourmet takes into account the processes of sugar generation and tries to predict a correct dose of insulin. In principle, these two approaches should be combined: it is very beneficial to have a device that is able to predict sugar variations based on understanding of real physiological processes rather than simply trying to immediately correct current sugar values. Such devise every time has a “pendulum” problem in practical realization.
5. Conclusions
- Available knowledge on diabetes type 1 and 2 is often not applicable to type 0 since the digestive system anatomy and physiology are dramatically different.Taking into consideration the causes and symptoms of different types of diabetes, it is proposed to classify the post-surgical cases as type 0 diabetes (total pancreatectomy or Whipple procedure). Such patients have a strongly modified digestive system and some new approaches in blood sugar monitoring, analysis, and treatment technologies should be developed. Although type 0 diabetic patients are the smallest group among the entire diabetic community, they are faced with significant challenges in their everyday life, and are in a great need of very specific guidelines in their daily health care. They also provide a unique opportunity to investigate the processes running in other types of patients with a partially or completely dysfunctional pancreas. The series of papers: “Insulin/Enzyme Calculator “Gourmet” for Type 0 Diabetic Patients” is a first step in the development of blood sugar control methods for type 0 diabetes patients. This is done through computer simulations of some stages of the digestion process and precise in-time monitoring of specific patient blood sugar behavior. This technology was developed to assist post pancreatectomy patients, especially those having undergone the Whipple procedure.
ACKNOWLEDGEMENTS
- The Gourmet series of papers was made possible thanks to the surgeon, Dr. Shiva Jayaraman, from St. Joseph’s Health Center, Toronto, ON, who has tremendous knowledge and practical experience in operating on Dr. Igor Peshko who was suffering from pancreatic cancer. In some sense, Dr. Peshko considers his surgeon to be the principle co-author of the paper. The non-medical person who practically supported this work was Igor’s wife, Nataliya. Sometimes, a lovely smile means much more than a ton of medications. Valuable contributions were provided by Dr. Olesya Peshko PhD, Bogdan Peshko, and Inderdeep Matharoo MSc, who were the first critical readers and editors of this paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML