-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2016; 5(5): 87-91
doi:10.5923/j.diabetes.20160505.01

Association of Post Prandial Hyper Triglyceridemia and Carotid Intima Media Thickness in Patients with Type-2 Diabetes Mellitus
P. Amruth Rao 1, P. Ramulu 2, K. Paul Marx 3, B. Tirumala Rao 2, Venkata Ramana Devi 3
1National Institute of Nutrition, Hyderabad, India
2Department of General Medicine, Osmania Medical College, Hyderabad, India
3Department of Biochemistry, University College of Science, Osmania University, Hyderabad, India
Correspondence to: P. Ramulu , Department of General Medicine, Osmania Medical College, Hyderabad, India.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Aim: To investigate the role of postprandial triglyceride levels and its relation with carotid intima media thickness in patients with type 2 diabetes mellitus. Methods: This is a case-control study which comprises 50 patients of age between 40 and 60 years with type 2 diabetes mellitus of more than or equal to 3 years duration. Carotid artery Doppler was done by B-mode ultrasonography using a 7.5MHz transducer. Blood samples were obtained after an overnight fast (12 hours) for total cholesterol, Fasting triglyceride, HDL, LDL, VLDL levels, which are measured by standard laboratory technique. Then patients ate a standard meal that had a total energy of 9 Kcal/Kg with 60-65% of this energy being supplied by carbohydrate, 15-20% by protein and 20% by fat after taking insulin or oral hypoglycemic agent. Blood samples were taken again 4 hours after the meal for postprandial triglyceride level. Results: Patients were divided into three groups. The first group normo-normo (NN) group i.e patients having normal fasting and postprandial triglyceride levels. Second group is normo-hyper (NH) group i.e patients having normal fasting but high postprandial triglyceride levels. Third group is hyper-hyper (HH) group i.e patients with high fasting and high postprandial triglyceride levels. Carotid intima media thickness was significantly higher in both the NH (1.78±0.81mm) and HH group (2.06±0.08mm) than the NN group (0.52±0.13mm). The correlation between CIMT and fasting triglyceride, postprandial hypertriglyceride was statistically significant (P<0.001). Conclusions: Postprandial hypertriglyceridemia, despite normal fasting triglyceride levels, may be an independent risk factor for early atherosclerosis in type 2 diabetics. Hence PPTG levels should be monitored in patients with type 2 diabetes.
Keywords: Postprandial hypertriglyceridemia, Carotid Intima–Media Thickness, Type 2 diabetes mellitus
Cite this paper: P. Amruth Rao , P. Ramulu , K. Paul Marx , B. Tirumala Rao , Venkata Ramana Devi , Association of Post Prandial Hyper Triglyceridemia and Carotid Intima Media Thickness in Patients with Type-2 Diabetes Mellitus, International Journal of Diabetes Research, Vol. 5 No. 5, 2016, pp. 87-91. doi: 10.5923/j.diabetes.20160505.01.
Article Outline
1. Introduction
- Diabetes is a group of metabolic diseases characterized by hyperglycaemia [1]. It is worldwide in distribution and the incidence of both type 1 and type 2 diabetes is rising dramatically. Globally, diabetes is one of the most common non-communicable diseases leading to mortality and morbidity in many developed countries. The worldwide prevalence of DM has risen significantly from as estimated 30 million cases in 1985 to 285 million in 2010 [2]. Based on current trends, 552 million individuals will have diabetes by the year 2030 [3] with majority of individuals in the most productive age group and elderly age of their lives.Diabetes is associated with the development of premature atherosclerotic vascular disease. The increased risk has been attributed to the high prevalence of multiple atherosclerotic risk factors among diabetic patients. Moreover, South Asians and Asian migrants are at unusually high risk for developing coronary artery disease (CAD) [4] and diabetes [5].Cardiovascular disease is increased from one to five fold in type 1 or type 2 DM individuals as stated by Framingham heart study. Further, American heart association designated that in patients with DM, cardiovascular disease (CVD) risk is substantially increased [6-10] which is the major cause of morbidity and mortality in type 2 DM [11, 12]. In fact, diabetes is considered as a coronary equivalent. Approximately 80% of all deaths and more than 75% of all hospitalization in patients with diabetes are due to CVD [13, 14].Risk factors specific to the diabetic population include microalbuminuria, gross proteinuria, serumcreatinine, abnormal platelet function, endothelial and vascular smooth muscle dysfunction. In addition to coronary artery diseases, the risk for cerebrovascular disease is increased in individuals with DM [15, 16]. On other hand factors factors such as dyslipidaemia and hypertension also are involved in the macrovascular complications [17].The dyslipidemia that accompanies type 2 diabetes plays an important role in the pathogenesis of accelerated atherosclerosis. Dyslipidemia features to have elevated very low density lipoproteins (VLDL), total triglycerides (TG’s) and decreased high density lipoproteins (HDL) concentration in the serum [18]. Several studies have proved that elevated triglyceride levels is a better predictor of Ischemic heart disease (IHD) than elevated LDL cholesterol levels and some studies highlighted coronary events increasing in patients with fasting triglyceride level > 1.13 mmol/L [19]. In the WHO multinational study, the single risk factor that correlated most closely with the occurrence of Coronary artery disease in diabetics was serum TG concentration. On finish of 7 years prospective study, high TG levels (>203) mg/dl) were associated with a 2-fold increase in risk for Coronary artery disease events in diabetics.It is believed that postprandial hypertriglyceridemia develops generally 3-6 hours after a meal. Further it goes elevated after next meal thus exposing the blood vessels most of the time to this postprandial phenomenon leading to atherosclerosis. Hence it is imperative to measure triglyceridemia in the postprandial state in diabetic patients. However, due to asymptomatic nature of atherosclerosis in less severe form, it is often reliable to measure CIMT as its surrogate non-invasive marker [20]. CIMT done by B-mode ultrasound visualizes the arterial walls and monitors the early stages of the atherosclerotic process [21]. Increased Carotid IMT is observed in type 2 diabetes [22] especially in postprandial hypertriglyceridemia stage [23-25]. Further, Increased Carotid IMT is associated with increased risk of Ischemic heart disease (IHD) and cerebrovascular disease (CVD) in diabetes [15, 16]. However there was a need to correlate postprandial triglyceride levels and carotid intima media thickness values in our study to find out whether postprandial hypertriglyceridemia, despite normal fasting triglyceride levels, serves as an independent risk factor for early atherosclerosis in type 2 diabetes.
2. Material and Methods
- This study was done on patients attending the Osmania General Hospital, Hyderabad. The total duration of the study was 2 years. This is a case-control study which included 50 patients of age between 40 to 60 years with type 2 diabetes mellitus of more than or equal to 3 years duration and all other patients with lipid abnormalities and history of hypolipidemic drugs, ischemic heart disease, cerebrovascular disease and peripheral vascular disease were excluded. Blood samples were obtained after an overnight fast (12 hours) for Lipid profile-total Cholesterol, HDL, LDL, triglycerides, VLDL. Then patients ate a standard meal that had a total energy of 9 Kcal/Kg with 60-65% of this energy being supplied by carbohydrate, 15-20% by protein and 20% by fat after taking insulin or oral hypoglycemic agent. Blood samples were taken again 4 hours after the meal for postprandial triglyceride level. Routine investigations like Fasting blood sugar, postprandial blood sugar were done as per the standard laboratory protocols and other investigations such as serum total cholesterol were performed by CHOD-POD method, triglycerides by GPO-POD method. B- mode ultrasonography of common carotid artery was done using a 7.5MHz transducer to assess the structural changes in the arteries.Friedewald’s formula:LDL cholesterol = Total serum cholesterol-(HDL cholesterol + TGL) mg/dl. VLDL is calculated from triglycerides which is one fifth if the serum triglycerides.
3. Statistical Analysis
- All results are expressed as mean ± SD. All analyses were evaluated with the help of statistical packages SPSS 17 version (SPSS, Inc., Chicago, IL, USA). A p value of less than 0.05 was accepted as statistically significant. Analysis of variance (ANOVA) has been used to find the significance of study parameters between NN, NH and HH.Informed consent: This study was approved by the Institutional Ethical Board of the Osmania Medical College. A written informed consent was sought after a complete description of the study narrated to each subject.
4. Results
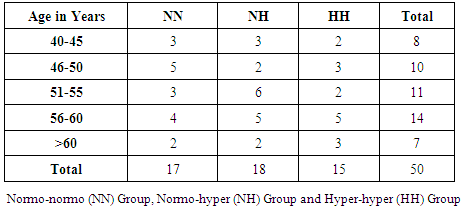
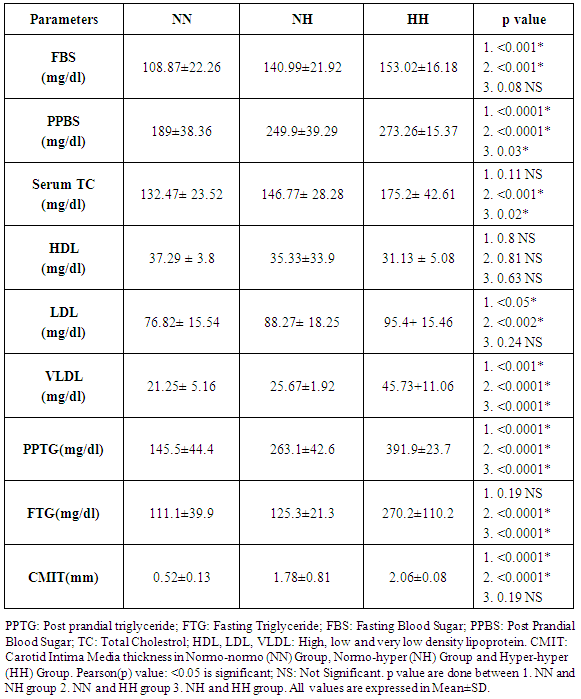
- In this study the mean age group of the subjects were 52.18 ± 6.30. Subjects were categorised in to three groups based on normal and elevated fasting triglyceride and postprandial triglyceride levels. NORMO-NORMAL (NN) GROUP: This group consists of subjects with normal fasting triglyceride level (≤150 mgs/dl) and normal postprandial triglyceride levels (≤200 mgs/dl). NORMO-HYPER (NH) GROUP: This group consists of subjects with normal fasting triglyceride level (≤150 mgs/dl) and elevated postprandial triglyceride levels (>200 mgs/dl). HYPER – HYPER (HH) GROUP: This group consists of subjects with elevated fasting triglyceride level (>150mgs/dl) and elevated postprandial triglyceride levels (>200 mgs/dl). The mean age of the patients in the NN, NH and HH group were 50± 7.06, 53.84± 3.89 and 52.40 ± 6.24 years respectively. Age wise distribution of patients among groups showed highest number in the age range between 56-60(28%) (Table 1). Out of total 50, Male patients were 16 and females 34. All the patients had history of diabetes for > 8 years. The mean duration of diabetes from initial diagnosis was 7.26±2.76 years.
|
|
5. Discussion
- In patients with type 2 diabetes mellitus, a significant postprandial hyper triglyceridemia and subsequent delay in clearing postprandial triglyceride following a standardized fast meal may result in a pro-atherogenic environment leading to atherosclerosis and macro vascular disease in type 2 diabetes subjects [18]. Even changes in mean levels of serum total cholesterol, serum HDL, serum LDL, serum fasting triglycerides are observed in patients with DM. Further, CIMT values also vary among different groups (NN, NH, HH) in diabetic patients. In a study done by Teno S et. al. (2000) the mean values for serum total cholesterol, serum LDL, serum fasting triglycerides, serum postprandial triglycerides were greater in the NH and HH group compared to NN group and decreased serum HDL in HH group. No much difference was found in mean CIMT values between NH (0.86±0.13 mm) and HH group (0.85±0.12mm) respectively. Our findings were in accordance to their study except for mean carotid IMT values in NH and HH group, where it showed 1.78±0.81 and 2.06±0.08mm respectively. Positive correlation existed between FTG, PPTG levels with CIMT. But PPTG levels were more strongly and independently correlated [23]. These observations were similar to our study. It is observed from this study that the mean CIMT in patients with postprandial hypertriglyceridemia (HH group) was significantly greater than that in patients with normal FTG and PPTG levels (NN group) (2.06 mm vs 0.52 mm, p<0.001). In conclusion, our result showed similarity to the study done by Ahmad J et.al (2003), where it was observed that CIMT was increased in patients with postprandial hypertriglyceridemia despite normal FTG levels, and the PPTG levels showed the strongest influence on CIMT [24]. Similarly; study done by ChenXiang et. al (2003), reflects the mean CIMT in patients with postprandial hypertriglyceridemia was greater than the patients with normal PPTG levels (0.90 mm vs 0.81 mm, p <0.05) [27].FTG and PPTG levels in diabetics were significantly higher than non-diabetics (p-value<0.01) as studied by Anirban Sinha in a correspondence communication submitted to JAPI (2012). Good correlation was found between FTG and PPTG in both diabetics and non- diabetics (Pearson r=0.54 and 0.70 respectively). PPTG correlated well with CIMT in diabetics (Pearson r = 0.55). Besides CIMT, cardiac risk was assessed by performing Electrocardiogram (ECG) and Treadmill Test (TMT) on study subjects. Their findings support that high PPTG carries significant risk for coronary artery disease in well controlled diabetics [28]. It is also worthwhile to note that CVD; which is the leading cause of deaths worldwide [29], demands for its early detection. This study used CIMT as a surrogative non-invasive quantitative marker for early atherosclerosis beside its use as a simple method for predicting future CVD risk [30]. Further, we have established CMIT direct correlation with PPTG by classifying our subjects in to three different groups mentioned earlier. Our findings revealed much similarity to other previous studies, in the patients with normal fasting triglyceride levels; a tendency was evident for the carotid intima media thickness to be greater when the postprandial triglyceride levels were high. Also, PPTG levels were more strongly and independently correlated with CIMT than FTG levels.
6. Conclusions
- In conclusion, the present study showed elevated fasting triglyceride level (FTG) significantly correlating with carotid intima media thickness, but higher correlation was found between elevated postprandial triglyceride level and carotid intima media thickness (CIMT). So, postprandial hypertriglyceridemia (PPTG) is better in risk assessing for an early atherosclerosis in type 2 diabetics patients than fasting triglyceride level (FTG).
ACKNOWLEDGEMENTS
- We acknowledge the Department of surgery, Faculty of Medicine of Osmania Medical College. The authors thank all technicians and nurses who helped in collecting the samples. The research team also thank all the patients for their co-operation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML