-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2016; 5(4): 63-69
doi:10.5923/j.diabetes.20160504.01

Camel Milk Ameliorates Hyperglycemia-Mediated Hyperlipidemia and Oxidative Stress on Streptozotocin-Induced Diabetic Rats
Adel Abdel Moneim1, Hamdi Helmy2, Eman S. Abdel-Reheim1, Wessam W Addaleel1
1Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Egypt
2Clinical Pathology Department, Faculty of Veterinary Medicine, Beni-Suef University, Egypt
Correspondence to: Adel Abdel Moneim, Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The present study aimed to assess the hypoglycaemic effects of camel milk (CM) and its efficiency on dyslipidaemia and altered antioxidant defence system in streptozotocin diabetic albino rats. The animals were divided into 2 categories; the pre-treated and the treated groups. RawCM (33 ml/kg b. w.) administered daily by oral cannula for 2 weeks pre and after diabetes induction for pre-treated group and 2 weeks only after diabetes induction for treated group. The current study revealed that camel milk administration in pre-treated and treated groups revealed marked significant amelioration on hyperglycaemia, hypoinsulinaemia, hyperlipidaemia, impaired antioxidant defence mechanisms and modulation of PPAR-γ and GLUT4 genes expression as compared to the untreated diabetic rats. These findings revealed the hypoglycaemic effects of camel milk against STZ-induced diabetes through potentiating insulin secretion, modulating PPARγ and GLUT4 expressions and also by hypolipidaemic, potent antioxidant effects.
Keywords: Camel milk, Hyperglycaemia, Hyperlipidaemia, Oxidative stress system, PPAR-γ and GLUT4 genes expression
Cite this paper: Adel Abdel Moneim, Hamdi Helmy, Eman S. Abdel-Reheim, Wessam W Addaleel, Camel Milk Ameliorates Hyperglycemia-Mediated Hyperlipidemia and Oxidative Stress on Streptozotocin-Induced Diabetic Rats, International Journal of Diabetes Research, Vol. 5 No. 4, 2016, pp. 63-69. doi: 10.5923/j.diabetes.20160504.01.
Article Outline
1. Introduction
- Diabetes was one of the first diseases described by an Egyptian manuscript from c. 1500 BCE mentioning "too great emptying of the urine" [1]. The number of people with diabetes has risen from 108 million in 1980 to 422 million in 2014 and the number of people with diabetes is expected to rise to 592 million by 2035 [2].For controlling diabetes, various treatments including diet, life style changes, biochemical and herbal medicine in combine or alone have been used [3]. The currently available antidiabetic drugs were including insulin secretagogues (sulfonylureas, meglitinides), insulin sensitizers (biguanides, metformin, and thiazolidinediones) and α-glucosidase inhibitors (miglitol, acarbose) [4]. On the other hand, for thousands of year's natural products have played a very important role in health care and prevention of diseases. The ancient civilizations of the Chinese, Indians and North Africans provide written evidence for the use of natural sources for curing various diseases [5]. Camel milk is gaining more popularity nowadays because of its high nutritional qualities and therapeutic value [6]. Camel milk contains several protective proteins including immunoglobulins, complements, lysozym, lactoferrin which considered the main iron-binding protein of milk, has biological activities. They have evaluated the potential of camel milk lactoferrin for its ability to inhibit the proliferation of the colon cancer cell line, HCT-116, in vitro, DNA damage and its antioxidant activities for the first time [7].Camel milk has insulin like activity, regulatory and immunomodulatory functions on β cells and exhibits hypoglycaemic effect when given as an adjunctive therapy, which might be due to presence of insulin/insulin like protein in it and possesses beneficial effect in the treatment of diabetic patients [8]. Moreover, camel milk consumption and lifestyle have definite influence on prevalence of diabetes. Hence, adopting such life pattern may play protective role in preventing diabetes to some extent [9]. The present study aimed to investigate the beneficial hypoglycaemic, hypolipidemic and antioxidant effects of raw camel milk on streptozotocin-induced diabetic rats.
2. Materials and Methods
- White male albino rats (Rattus norvegicus) weighting about 110-150g were used as experimental animals in the present investigation. They were supplied from the animal house of Research Institute of Ophthalmology, El-Giza, Egypt. They were kept under observation for 10 days before the onset of the experiment to exclude any inter current infection. The chosen animals were housed in metal (stainless steel) cages at normal atmospheric temperature (25±5°C) as well as 12 hrs daily normal light periods. All the procedures were performed in accordance with the Institutional Animal Ethics Committee in Beni-Suef University recommendations.Raw camel milk (CM) is opaque white in colour with normal odder and salty taste. It was administered in a dose of 33 ml/kg body weight for each rat daily by oral cannula [10]. Composition of raw camel milk analysis indicated 34.4 ± 2.8 g.l-1 fats, 33.1 ± 2.1 g.l-1 proteins, 45.1± 3.1 g.l-1 lactose, 8.15 ± 0.15 g.l-1 ashes, 6.57 pH and 873.7 g.l-1 moisture. Examination of the composition of camel’s milk proteins revealed 74.1% of casein and 25.9% of whey proteins of the total content. Camel milk samples were collected from a camel farm in Marsa Matrouh, Western desert, Egypt. All lactating camels consumed the same type of food. The milk was collected in the morning in sterile screw bottles and kept on ice during transportation to the laboratory where milk bottles were stored at 4°C.
2.1. Induction of Diabetes and Animals Grouping
- Diabetes mellitus was experimentally induced in animals fasted for 16 hrs by intra-peritoneal injection of 45 mg/kg b.wt. streptozotocin purchased from Sigma (Sigma-Aldrich Co., St. Louis, Missouri, USA) dissolved in citrate buffer, pH 4.5 [11]. Ten days after streptozotocin injection, rats were screened for blood glucose levels. Overnight fasted (about 12 hrs) animals were given glucose (3 g/kg b.wt.) by gastric intubation. After 2hrs of oral administration, blood samples were taken from lateral tail vein, centrifuged and serum glucose concentration was measured. Rats having serum glucose ranging from 200-300 mg/dl (mild diabetes), after 2 hrs of glucose intake, were included in the experiment, while the others were excluded. For pre-treated groups: 2 weeks after administration of CM; diabetes mellitus was experimentally induced in animals fasted for 16 hrs by intra-peritoneal injection of 45 mg/kg b.wt. streptozotocin purchased from Sigma dissolved in citrate buffer, pH 4.5.The animals (28 ones) were divided into 2 categories; the prophylactic groups (which administered TQ before diabetes induction) and the treated groups (which administered TQ after diabetes induction), and were divided into four groups (7 animals / group):Ÿ Group 1: The first group of normal animals were kept without treatments under the same laboratory condition and regarded as frank (normal control) group for the all groups.Ÿ Group 2: The second group was regarded as diabetic control for all groups and kept after diabetes induction without treatments under the same laboratory condition for 2 weeks.Ÿ Group 3: The third group was given camel milk (CM) by a dose of 33 ml/kg body weight for each rat daily by oral cannula for 2 weeks before streptozotocin injection and for another 2 weeks after (pre-treated-CM group).Ÿ Group 4: The fourth group was given Camel Milk (CM) by a dose of 33 ml/kg body weight for each rat daily by oral cannula for 2 weeks after streptozotocin injection (treated-CM group).By the end of the experimental period, normal, diabetic control, pre-treated and diabetic treated rats were sacrificed under mild diethyl ether anaesthesia. Blood samples were taken and centrifuged at 3000 r.p.m. for 30 minutes. The clear, non-haemolysed supernatant sera were quickly removed, divided into four portions for each individual animal and kept at –20°C for further analysis. Also small pieces of liver were excised and used fresh for oxidative stress determinations.
2.2. Determination of Biochemical and Molecular Assays
- OGTT test was performed on normal, diabetic control, prophylactic and diabetic treated after treatment with TQ. Tail vein blood samples were obtained from over-night fasted animals. Successive blood samples were then taken at 30, 60, 90 and 120 minutes following the administration of 3 g glucose /kg b.wt. Glucose was dissolved in distilled water and given by gastric intubation. Blood samples were centrifuged and serum was obtained for determination of glucose concentration according reagent kit obtained from Biochon (Germany) through Alkan medical agent. Otherwise, serum insulin was determined with radioimmuno-assay kit obtained from DPC (Diagnostic Products Corporation), Los Angeles, U.S.A. (coat-A-count). On the other hand, serum cholesterol, HDL-cholesterol and triglycerides concentrations were estimated according reagent kit purchased from Reactivos Spinreact Company, Spain. Otherwise, LDL-cholesterol and vLDL- cholesterol levels were calculated according to Friendewald [12] formula. However, malondialdehyde (MDA; lipid peroxidation marker) concentration was determined in the liver tissue homogenate according to the method of Yagi [13]. Otherwise, nitric oxide (NO) was determined by NO-reagent kit (Biodiagnostic, Egypt). So, superoxide dismutase (SOD) was estimated according to the method of Kakkar et al. [14] while, glutathione peroxidase (GPX) was assessed by the method of Wendel [15]. Furthermore, glutathione S-transferase (GST) and reduced glutathione (GSH) were assessed by the method of Habig et al. [16] and Moron et al. [17], respectively.Real time quantitative polymerase chain reaction (qPCR) differs from regular PCR by including in the reaction fluorescent reporter molecules that increase proportionally with the increase of DNA amplification in thermocycler. There are two types of fluorescent chemistries for this purpose: double strand DNA-binding dyes and fluorescently labelled sequence specific probe/primer. SYBR Green I dye and TaqMan® hydrolysis probe are the common examples for these two respectively. Total RNA was extracted from the visceral adipose tissue of each rats using TriFastTM reagent (PeQlab, Germany). RNA was purified and spectrophotometrically quantified. The produced cDNA was amplified using Go Taq green master mix (Promega, USA) using the following sets of primers; 5′-ACATACCTGACAGGGCAAGG-3′ (forward) and 5′-CGCCCTTAGTTGGTCAGAAG-3′ (reverse) for glucose transporter type 4 (GLUT4) and 5′-GCCCTTTGGTGACTTTATGGA F-3′ (forward) and 5′-GCAGCAGGTTGTCTTGGATG-3′ (reverse) for PPAR-γ. PCR was performed using Green master mix (Promega, USA) and T100TM thermal cycler (Bio-Rad Laboratories, USA) under the following conditions: initial denaturation at 95°C for 5 min, 35 cycles set at 94 °C (1 min) for denaturation, 55°C (1 min) for annealing and 72°C (1 min) for extension, and finally at 72°C (5 min) to complete the extension reaction. PCR products were subjected to electrophoresis on 1.5% agarose gels containing ethidium bromide [18]. Images from electrophoresed gels were captured by a camera in a computer assisted gel documentation system. Relative band intensities of each sample were calculated after being normalized with the band intensity of β-actin using Phoretix 1-D densitometry software v.11 (TotalLab Ltd., UK) and values presented as % mRNA relative to control.
2.3. Statistical Analysis of Results
- The Statistical Package for the Social Sciences (IBM SPSS for WINDOWS 7, version 22; SPSS Inc., Chicago) was used for the statistical analysis. Comparative analysis was conducted by using the general linear models procedure (IBMSPSS). Values of P>0.05 were considered statistically non-significant, while values of P<0.05 were considered statistically significant. The results were expressed as mean ± standard deviation (SD) and values of P>0.05 were considered statistically non-significant, while values of P<0.05 were considered statistically significant.
3. Results
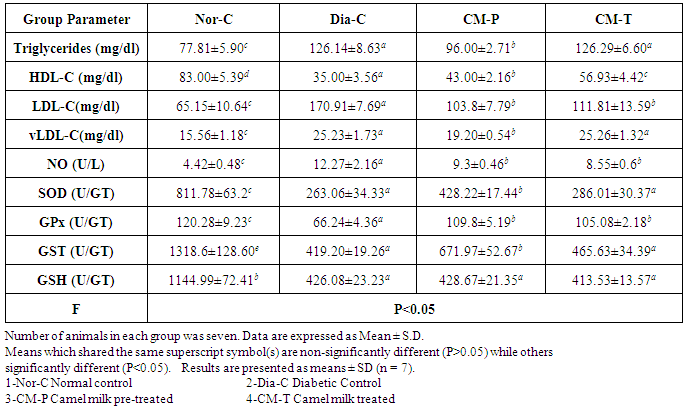
- The results of the current study revealed that the untreated diabetic rats showed noticeable significant (P<0.05) increase in fasting serum glucose level compared to normal control group, while the OGTT of both pre-treated and treated groups showed significant (P<0.05) hypoglycaemic effect after; 30min, 60min, 90min and 120 min of glucose administration with special respect to camel milk pre-treated which showed a marked close pattern to the normal group (Tab. 1 & Fig. 1A).
|
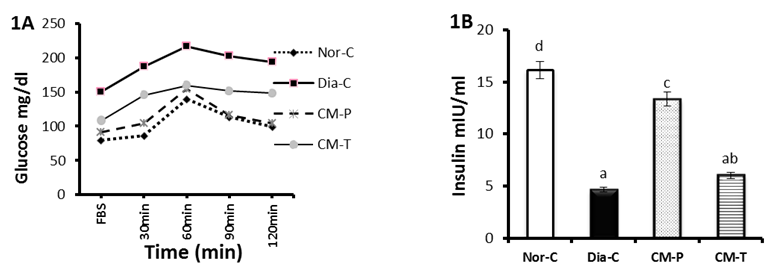 | Figure 1(A-B). Represent oral glucose tolerance (A) test (OGTT) and fasting serum insulin (B), of normal, diabetic control, camel milk pretreated and treated groups |
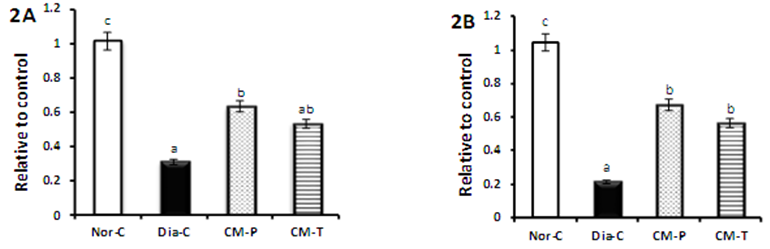 | Figure 2(A-B). Represent PPAR-γ (A) and GLUT4 (B) genes expression of normal, diabetic control, camel milk pretreated and treated groups |
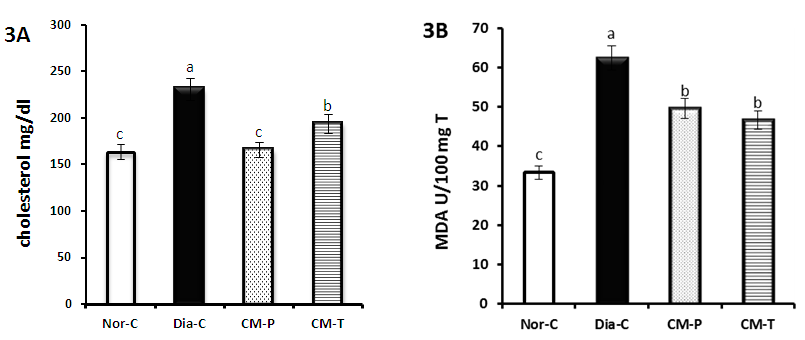 | Figure 3(A-B). Represent fasting serum total cholesterol (A) and lipid peroxidation marker (MAD) (B) of normal, diabetic control; camel milk pretreated and treated groups |
4. Discussion
- Diabetes mellitus (DM) is a global health concern which results in disturbances of carbohydrate, fat and protein metabolism [19]. Camel milk is known for its medicinal properties since ancient times and recently camel milk has been deeply studied for its special properties. Beneficial components such as lactoferrin, immunoglobulins, lysozyme and vitamins are in good quantity in camel milk [20]. Moreover, camel milk is different from other ruminant milk as it is low in cholesterol, sugar and protein but high in minerals, vitamins and contains insulin and immunoglobulins [21]. In the current study, we observed in diabetic rats significant increase in blood glucose level along with significant decrease in serum insulin level. This may be due to the destruction of pancreatic beta cells by STZ, reinforcing the fact that STZ induces diabetes, probably through the generation of oxygen free radicals [22]. Our results showed a significant improvement of insulin level in both pre-treated and treated camel milk group. These results are in accordance with the findings of Wang et al. [23] which have respectively shown that 14-week and three-month camel milk consumption had improved insulin levels of patients with T2DM [24]. Recently reports suggest that; insulin in camel milk possesses special properties that make absorption into circulation easier than insulin from other sources or cause resistance to proteolysis; camel insulin is encapsulated in nanoparticles (lipid vesicles) that make possible its passage through the stomach and entry into the circulation [25]. Camel Milk was found to contain approximately 52micro unit/ml insulin and it may be the reason for a lesser requirement of insulin in diabetic patients receiving camel milk [8]. The lack of coagulum formation of camel milk may act as an effective vehicle to take the milk insulin unchanged to the intestine, and from that it can be absorbed even if some of it is destroyed in the passage [26].Consequently, improved insulin level leads to a significant amelioration of oral glucose tolerance test (OGTT) of both CM groups especially that of prophylactic camel milk group which showed a very close pattern to normal control group more than that of treated camel milk group. Agrawal et al. [27] reported the low prevalence of diabetes in Raica community of Rajasthan, India and attributed the low prevalence level to consumption of camel milk. The low prevalence of impaired fasting glucose, impaired glucose tolerance and diabetes in camel milk consuming groups is due to various factors like high concentration of insulin/insulin like substance in camel milk. Although, patients treated with camel milk needed less insulin to achieve better control than the controlled group [26]. In type-2 diabetic animals; camel milk reduces FBS and post-prandial glucose. Area under curve-glucose (AUC-glucose) also decreased significantly along with HOMA-IR. It shows hypoglycaemic effect of camel milk reducing insulin resistance [9]. In addition, Al-Numair, [28] reported that treatment with camel milk restored the plasma glucose and insulin levels to near normal in streptozotocin-type 2 diabetic rats. The results of our study revealed significant decreases in peroxisome proliferator-activated receptor γ (PPAR-γ) expression and glucose transporter-4 (GLUT4) expression in diabetic group. These results are in accordance with data reported by Ahmed et al. [29]. It is well known that adipose tissue plays a crucial role in antidiabetic action of PPAR-γ. Moreover, PPAR-γ can directly regulate the genes responsible for glucose homeostasis. In addition, PPAR-γ can directly affect liver and pancreatic β-cells to improve glucose homeostasis [30]. GLUT4 is the insulin-regulated glucose transporter found primarily in adipose tissues and striated muscle (skeletal and cardiac). At the cell surface, GLUT4 permits the facilitated diffusion of circulating glucose down its concentration gradient into muscle and fat cells. Once within cells, glucose is rapidly phosphorylated by glucokinase in the liver and hexokinase in other tissues to form glucose-6-phosphate, which then enters glycolysis or is polymerized into glycogen [31]. GLUT4 deficiency resulted in decreased levels of lactate and FFAs in both the fasting and fed states, and of β-hydroxybutyrate in the fasting state. Since the disruption of one allele of GLUT4 led to severe peripheral insulin resistance and varying levels of GLUT4 protein in striated muscle and adipose tissue can markedly alter whole-body glucose disposal [32]. Meanwhile, our results confirmed that, in camel milk groups, PPAR-γ and GLUT4 expression significantly improved as compared to diabetic ones. It means that camel milk administration caused amelioration in diabetic conditions compared to the untreated diabetic rats via up-regulation of diabetes related gene expression, activation of the expressed genes, increasing the availability of insulin and its sensitivity, decreasing its resistance and increasing glucose transporting through the cell membrane. Diabetes is associated with profound alterations in lipid profile and an increased risk of cardiovascular disease [24]. Similarly, our results showed significant increases in total cholesterol (TC), triacylglycerol (TG), low density lipoprotein (LDL) and very low density lipoprotein (vLDL) of diabetic group compared to normal control group. However, HDL-C decreased significantly as compare to normal control group. This result agreed with that of Arkkila et al. [33] who found that the abnormalities in the lipid metabolism may be due to insulin deficiency. The present study illustrates that in pre-treated and treated groups of camel milk, the levels of TC, TG, LDL and vLDL decreased significantly as compared with diabetic group and this was associated with a significant increase in HDL-C in these groups. This was associated with a significant increase in HDL-C especially in CM treated group. Wang et al. [23] reported that camel milk had TC and TG lowering properties in type 2 diabetic rats. Our study concluded that control diabetic group animals showed enhanced oxidative stress, as evidenced by increased MDA and NO levels. This could be related to the beta-oxidation of fatty acids in hepatic steatosis, which stimulates reactive oxygen species (ROS) generation, lipid peroxidation, hepatocyte necro-inflammation, and apoptosis [34]. Moreover, current data on diabetic rats are associated with decreased GSH levels and SOD, GPX and GST activities. Concerning the present results, in the pretreated and treated groups, CM significantly reduced the liver tissue levels of malondialdehyde (MDA) and nitric oxide (NO) as compared with diabetic group. These results are in agree with Argo et al., who concluded that the antioxidant effect of CM plays a role in the reduction of hepatic fat accumulation and decreases systemic and hepatic oxidative stress as evidenced by increased decreased MDA production confirming that CM is potent antioxidant [35]. In parallel, in pretreated group, CM ameliorated significantly the reduced liver tissue levels of superoxide dismutase (SOD glutathione peroxidase (GPx) and glutathione S-transferase (GST) as compared with diabetic group. These results are in agreement with Al-Asmari et al. [36] who demonstrated that, camel milk helped to improve the diabetes-induced oxidative stress through amelioration of enzymatic superoxide dismutase (SOD) activity. This significant increase in GPx activity in diabetic rats treated with camel milk may have been a response to the increased peroxidative stress from the high SOD activity which converts O2 - into H2O2. Moreover, administration of CM revealed significance up-regulation of GST activity [36]. Also, El-Bahr, [37] reported that, camel milk augmented the antioxidant status via up-regulation of SOD, GPX and GST gene expression. Moreover, the antioxidant capacity of Lactoferrin (Lf), the main iron-binding protein of milk, was evaluated by different assays, including ferric-reducing/antioxidant power assay (FRAP), free radical-scavenging activity (DPPH), nitric oxide (NO) radical-scavenging assay, total antioxidant activity and DNA damage, compared with vitamin C and rutin [7]. It has been reported that camel milk contains high levels of vitamins (A, B2, C and E) and is rich in magnesium and Zinc [38]. More than 300 enzymes require Zn for their activity and it has a relationship with many enzymes in the body and can prevent cell damage through activation of antioxidant system [39]. Removal of free radicals by camel milk prevents tissues damage including the β-cells of the pancreas [21]. Therefore, the protective effects of camel milk could attribute to its antioxidant activity and it may possibly have chelating effects on toxicants [40].
5. Conclusions
- Therefore, camel milk exerted its ameliorative hypoglycaemic effect against STZ-induced diabetes by its own insulin/insulin-like components, hyperinsulinemic effect and modulating the gene activity and gene expression levels. The different mechanisms by which CM can attenuate the diabetic conditions in addition to and its ability to modulate the oxidative stress with augmenting antioxidant defence system, is the hypolipidemic effects.
ACKNOWLEDGEMENTS
- We would like to thank Professor Gamal Morsy, Faculty of Science, Cairo Univesrsity, Egypt for their fruitful directions and helping in statistical analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML