-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2016; 5(3): 41-47
doi:10.5923/j.diabetes.20160503.01

The Prickly Pear Cactus (Opuntia Fiscus-Indica) Cladode Extracts Modulate Blood Sugar in Swiss White Albino Mice
Peris Moraa Mokua1, Oscar Mayunzu2, Meshack Obonyo1, John Thuita3, James Mutuku3, Jane Rutto3, Grace Murilla3
1Department of Biochemistry and Molecular Biology, Egerton University, Egerton, Kenya
2Kenya Forestry Research Institute, Forest Product Research Centre, Karura, Mobile Plaza, Nairobi
3Kenya Agricultural and Livestock Research Organization (KALRO) -Biotechnology Research Institute, Kikuyu, Kenya
Correspondence to: Grace Murilla, Kenya Agricultural and Livestock Research Organization (KALRO) -Biotechnology Research Institute, Kikuyu, Kenya.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background and purpose: Medicinal plants including the prickly pear cactus have been reported to modulate blood sugar levels. Extracts of prickly pear cactus have been used in various parts of the world to manage diabetes mellitus. However the cactus is viewed as a weed in Kenya. The current study therefore aimed at evaluating the efficacy of prickly pear cactus cladode extracts from Kenya in managing diabetes mellitus in induced mice. Methods: Healthy, adult Swiss white albino male mice weighing 20-30g were induced with diabetes mellitus using Alloxan (150 mg/kg body weight) administered intra-peritoneally. Prickly pear cactus cladode extracts were administered at daily dosage of 0.6 ml for pre-determined periods. Fasting blood sugar levels and live body weights were monitored at intervals of 72 hours throughout the experimental period of 30 days. Results: Alloxan administration resulted in about 3- to 4-fold increase in blood sugar levels. Treatment of diabetic mice with cactus cladode extracts led to decline in blood sugar levels of the animals, however, the levels varied with the period of treatment. Diabetic animals treated with cactus cladode extracts for 10 days showed a significant decline in blood sugar levels on the 7th (p=0.012) and 10th (p=0.001) days of feeding on the extracts when compared to the positive control (diabetic, not treated) animals. Conclusions: This study has demonstrated that extracts of prickly pear cactus cladodes from Kenya have potential in managing blood sugar in diabetic mice.
Keywords: Alloxan, Opuntia, Prickly pear cactus, Cactus cladodes, Diabetes
Cite this paper: Peris Moraa Mokua, Oscar Mayunzu, Meshack Obonyo, John Thuita, James Mutuku, Jane Rutto, Grace Murilla, The Prickly Pear Cactus (Opuntia Fiscus-Indica) Cladode Extracts Modulate Blood Sugar in Swiss White Albino Mice, International Journal of Diabetes Research, Vol. 5 No. 3, 2016, pp. 41-47. doi: 10.5923/j.diabetes.20160503.01.
Article Outline
1. Introduction
- Diabetes mellitus is considered one of the major public health concerns globally [1] and ranks 4th among non-communicable diseases [2] because its effects reach multiple organs causing serious heath complications ranging from brain damage, cardiovascular diseases, renal failure and death [3]. The disease is characterized by hyperglycemia associated with abnormal lipid, protein and carbohydrate metabolism [4]. Two major types of diabetes mellitus have been characterized by the World Health Organization based on their morbidity and mortality rates: Type I diabetes mellitus, which accounts for 10% and Type II that accounts for 90% of all the diabetic cases in the world [5] [6] [7]. Type 1 diabetes is often caused by a deficiency in insulin production while Type II occurs as a result of ineffective use of insulin by the body [8]. Studies by the World Health Organization and International Diabetes Federation indicate that the number of people suffering from diabetes across the world has increased significantly over recent years. For instance, in the year 2000,171 million cases of diabetes were recorded worldwide [9]. Recent studies show there are 382 million diabetic patients globally. This figure is likely to increase to 592 million by the year 2035. This will represent a prevalence of 10.1%, an increase from 8.3% in 2013 [10].The management of diabetes mellitus is a lifelong process that requires constant medication and specialized health care [11]. While people with type 1 diabetes mellitus require regular insulin intake, a lifestyle change to include healthy diet and physical exercise is key to managing type 2 diabetes mellitus. Two major classes of oral drugs are prescribed for diabetic patients because of their affordability and mild side effects: the biguanides such as metformin and phenformin and the sulphonylureas such as gilbenclamide, diabenese, orinase and glucotrol Amaryl [12]. Other classes of oral pills include thiazolidinediones, alpha-glucosidase inhibitors, glucagon like peptides-1(GLP-1) receptor agonists and megilitinides. While these approaches are promising in management of diabetes mellitus, they are costly and can pose severe side effects to the patients. This challenge has, therefore, encouraged the use of herbal therapy including the use of prickly pear cactus extracts in managing diabetes mellitus. Prickly pear, which is also known as Opuntia, is a cactus that belongs to the Cactaceae family. Opuntia is native to Mexico but is also spread widely in most semi-arid countries [13]. However, its occurrence in Africa is due to the introduction of exotic seeds [14]. Extracts of this plant have been used to treat various ailments including diabetes mellitus [15] [16]. However, in Kenya, it is viewed as a weed that grows in semi-arid areas where it is used as a live fence for protection of agricultural lands against invasion by grazers as well as marking boundaries and fence homesteads to protect animals from predators [17]. So far, there has not been any assessment of the anti-hyperglycemic potential of prickly pear cactus in Kenya. The present study therefore assessed the efficacy of prickly pear cactus cladode extracts in controlling blood sugars of alloxan-induced diabetic mice. The cladodes were selected as a sustainable source of raw material due to their availability throughout the year in the arid areas.
2. Materials and Methods
- The prickly pear cactus plant samples used in this study were collected from Baringo County of Kenya. Pads (cladodes) were pruned and placed in sterile sampling bags, sealed and stored in cool boxes containing ice cubes. The plant samples were then transported to Kenya Forestry Research Institute (KEFRI) laboratories for preparation and National Museums of Kenya for identification. The cactus plant used in this study was identified as Opuntia fiscus. Materials were cut into small pieces, blended, filtered, concentrated to a third of the total volume [18] and stored at -20°C until required. Adult healthy Swiss White albino male mice weighing 20-30g, housed under natural light at 21-24°C were randomly allocated to different mice cages, and de-wormed using Ivermectin (Shanghai Gongyi Veterinary Medicine Plant, Huancheng East Road, China). The animals were maintained on a standard feed (Unga Ltd, Kenya) and water ad libitum. They were acclimatized to laboratory environment for two weeks before the start of the experiments. Experiments were performed as per guidelines set by the Institutional Animal Care and Use Committee of Biotechnology Research Institute.Diabetes was induced in the animals by administration of a single dose of alloxan monohydrate (Sigma-Aldrich, St. Louise, MO, USA) at a dose of 150 mg/kg body weight [19]. After 3 days, the mice were fasted overnight and fasting blood glucose (FBG) levels measured using SD CodeFreeTM glucometer (SD Biosensor, Inc. Korea) with compatible test strips. Animals with FBG levels above 200 mg/dl [20] were considered diabetic and used in the study. In order to determine the optimal volume of extract for hypoglycemic study, four groups of three alloxan-induced diabetic mice were orally given a single dose of cactus extract at either 0.2 ml, 0.4 ml, 0.6 ml or 0.8 ml, and their blood sugar levels monitored for 24 hours. Administration was by oral gavage.For the hypoglycemic experiments, four groups of 6 mice each were used: Group 1 (negative control, not induced with diabetes, not fed on extract), Group 2 (positive control, induced, not given extract), Group 3 (Induced, given daily dose of 0.6 ml extract for 5 days), and Group 4 (Induced, given daily dose of 0.6 ml extract for 10 days). Baseline blood glucose levels of all the animals were taken before oral treatment with cactus cladode extract. FBG levels (mg/dl) were determined at intervals of 72 hours during and for 15 days after the administration of cactus cladode extract [21]. All the animals were fasted overnight before blood collection, during which time, they had free access to water.Live bodyweights of all experimental animals were determined at 3-day intervals during the two-week acclimatization period and throughout the observation period of 30 days following administration of the extract. All data were expressed as mean ± standard error of the mean (mean±SEM). One way analysis of variance (ANOVA) followed by Turkey post hoc tests was performed to determine whether the differences of means between the treatment groups and positive control group were statistically different. P values of <0.05 were considered to be statistically significant. Analysis of the data was performed using SPSS software (IBM Inc. version 20.0).
3. Results
- A three- to four-fold increase in blood sugar levels was recorded following induction of mice with diabetes. The lowest mean (±SE) level of blood sugar before induction of mice with diabetes was 126.3±8.7 mg/dl and the highest was 140.7±11.25 mg/dl. Following induction, these values were 317.3±20.90 mg/dl and 495.0±20.17 mg/dl, respectively. No decline in blood sugar levels was observed in animals that were treated orally with 0.2 and 0.4 ml cactus extract. However, 66.7% of diabetic animals died within 12 hours following oral administration of 0.8 ml cactus cladode extract. Animals given 0.6 ml extract showed a decline in mean (±SE) blood sugar levels from 477.6±15.13 mg/dl to 420.9±16.70 mg/dl 24 hours after feeding on cactus cladode extract. Based on these findings, 0.6 ml of the cactus cladode extract concentrate was selected as the optimal dose for the hypoglycemic study.
3.1. Hypoglycaemic Activity Test
- The negative control animals (Group 1) had mean (±SE) value blood sugar fluctuating from 137.2±5.96 mg/dl to 172±9.93 mg/dl. These values were generally maintained throughout the observation period of 30 days. Approximately 53% of all the animals given alloxan in Groups 2, 3 and 4, developed diabetes mellitus (blood sugar levels above 200 mg/dl) after 24 hours and maintained the diabetic state for 72 hours. Positive control (Group 2) animals had blood sugar levels of 164.9±4.98 mg/dl before the administration of alloxan. These levels were elevated to 413.3±43.64 mg/dl 72 hours after the administration of alloxan. This group recorded gradual increase in mean (±SE) blood sugar levels throughout the study attaining levels of 423.4±41.38 mg/dl at the end of the observation period of 30 days. Group 3 animals had mean (±SE) blood sugar levels of 138.0±8.88 mg/dl before administration of alloxan which increased to 451.2±40.07 mg/dl 72 hours after administration of alloxan. A decline in mean (±SE) blood sugar levels was observed in this group throughout the 5 days period of feeding on extract. However, this decline was not statistically different (p=0.638) when compared to the positive control group. Group 4 recorded mean (±SE) blood sugar level of 140.2±10.95 mg/dl before administration of alloxan. An elevation to 484.5±20.61 mg/dl was recorded in this group following injection with alloxan. A significant decline (p=0.012 and p=0.001) in mean (±SE) blood sugar levels was observed in this group on the 7th and 10th days following administration of extract (Table 1).
|
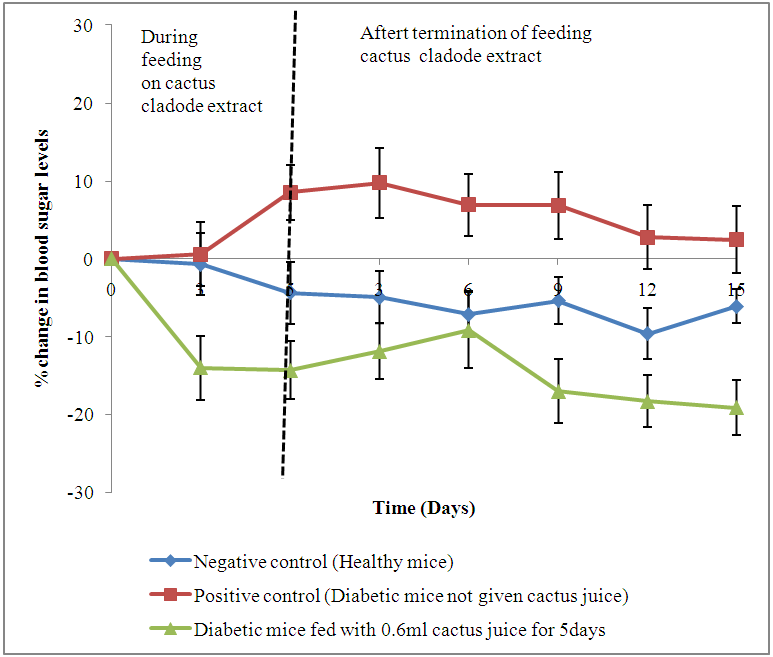 | Figure 1. Changes (%) in blood sugar levels of diabetic animals fed on cladode extract daily for a period of five days days |
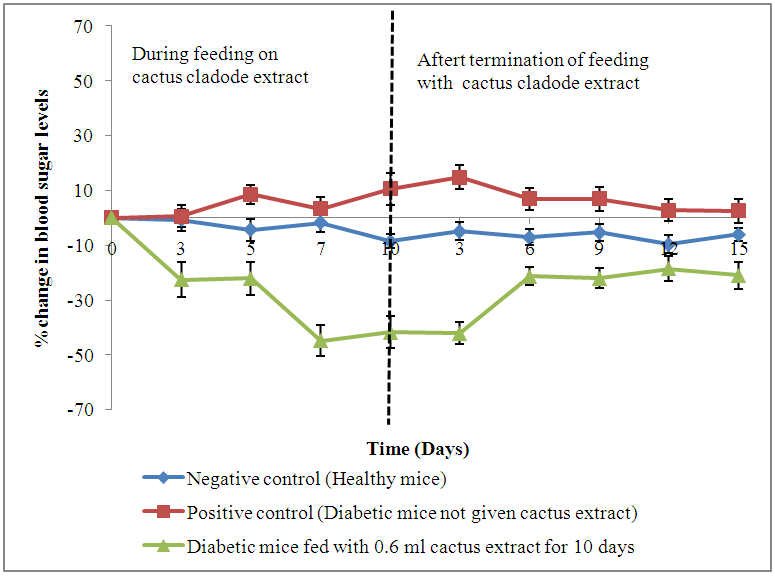 | Figure 2. Changes (%) in blood sugar levels of diabetic animals fed on cladode extract daily for a priod of 10 days |
3.2. Live Body Weights
- Results showed a gradual increase in bodyweights of the negative control animals throughout the experimental period of 30 days. The alloxan-induced diabetic animals recorded a decline in live body weights of 9.4% (Group 2), 6.7% (Group 3) and 11.2% (Group 4) following administration of alloxan. All the animals showed a gradual decline in body weights during feeding with cactus extract, with the highest drop recorded on days 5 and 10 post feeding. The positive control group recorded a drop in mean (±S.E) throughout the observation period from 26.3±0.64 g to 24.4±0.72 g. Diabetic animals on 0.6 ml cactus cladode extract for 5 days showed the highest decline (10.8%) in body weight at the end of treatment period. However, an increase in body weights was observed in this group when administration of extract was terminated (Figure 3).
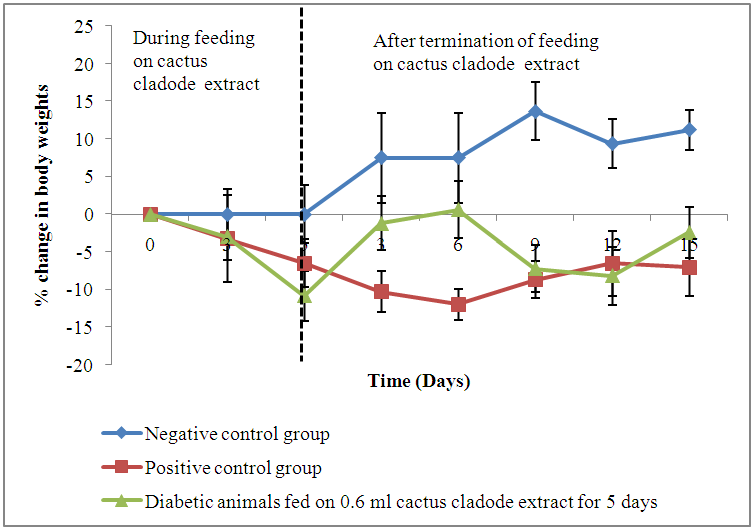 | Figure 3. Changes (%) in live body weights of diabetic mice fed on cactus cladode extract daily for a period of 5 days |
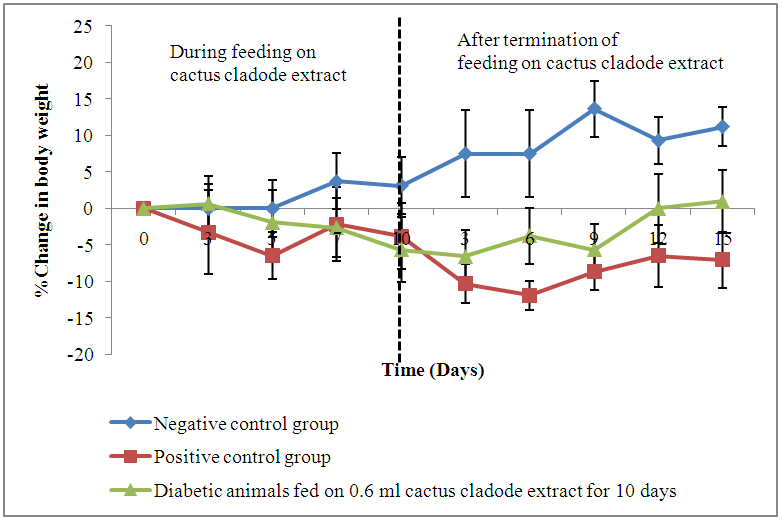 | Figure 4. Change (%) in live body weights of diabetic mice fed on cactus cladode extracts daily for a period of 10 days |
4. Discussion
- The findings of this study show that the diabetic status (blood sugar levels above 200 mg/dl) was achieved by administration of alloxan to mice. Alloxan monohydrate is known to induce experimental diabetes by selective destruction of the insulin-producing pancreatic beta cells of islet of Langerhans, which results in the reduction of insulin levels and increased blood glucose eventually leading to diabetes mellitus [1] [22]. Our results also demonstrated that extracts of Opuntia fiscus-Indica cladodes from Kenya significantly reduced blood sugar levels in experimental diabetic mice when administered orally at a volume of 0.6 ml daily for a period of 10 days compared to 5 days, thereby showing its potential in the management of diabetes. These results corroborate those of Meckes-Lazyoa and Roman-Ramos who reported significant reductions in serum glucose levels of non-insulin dependent patients given Opuntia streptacantha cladode extracts for 10 days [23]. In another study, Frati et al gave crude extracts of Opuntia streptacantha Lemaire to one group of type 2 diabetes mellitus patients and to the other group of patients he gave boiled stems of Opuntia streptacantha Lemaire. He, however, reported an insignificant decrease in blood sugar levels of non-insulin dependent patients that received crude extracts compared to those who received boiled stems of Opuntia streptacantha Lemaire [24]. Our findings suggest that Opuntia fiscus-Indica cladodes from Kenya can cause hypoglycemic effects when consumed in their crude form. We also reported a decline in blood sugar levels of the diabetic treated animals following termination of feeding on cactus cladode extracts. However, the decline in blood sugar levels of the animals was not significant when compared to the positive control, which also suggests that a daily intake of cactus cladodes extracts is required to maintain the hypoglycemic effect and that the plant could also be consumed as a cooked vegetable. Prickly pear cactus cladodes are believed to contain pectin, which are soluble fibers that interfere with carbohydrate metabolism [25] [26], stabilize the movement of intestinal food and slow down the absorption of glucose [27], which in turn reduce blood sugar levels [28] [29]. The anti-hyperglycemic effect observed from our results may be attributed to pectin and other insulin-like compounds present [30] in the cladodes that may have enhanced the sensitivity of pancreatic β-cells stimulating them to produce more insulin [31] and enhance insulin activity, which eventually led to improved glucose utilization by the cells [32] [27] and the reduced blood sugar levels. The anti-hyperglycemic effect observed could also be attributed to phenols and flavonoids that have been reported in prickly pear cactus cladodes [33] [34]. Phenols have been reported to improve sensitivity of the tissues to insulin through their ability to scavenge free radicals [35] [36]. Flavonoids, on the other hand, are known to promote the uptake of glucose by peripheral tissues [36] thus reducing the glucose levels in blood.The alloxan-induced untreated diabetic mice recorded a consistent drop in their mean body weights throughout the observation period whereas the negative control group showed a gradual increase in their body weights. These results are in agreement with those obtained by El-Razek and Hassan [26] using Opuntia fiscus-Indica fruit extract. The loss in body weight observed may have been as a result of muscle wasting due to the lack of carbohydrates for utilization as an energy source that arises from poorly controlled diabetes mellitus [37]. Diabetic animals fed on Opuntia fiscus-Indica cladode extract recorded a decline in their body weights during the study period. However, the animals showed an increase in body weight when the administration of cactus cladode extracts was stopped. The changes in body weights observed were, however, not significant compared to those of the diabetic (positive) control group. The observed weight loss could be attributed to the high content of dietary fibers that have been reported in prickly pear cactus cladodes [39]. Dietary fibers promote weight loss through reducing energy intake by binding to dietary fats making them unavailable for use in digestion [40]. It is also believed that the dietary fiber in the cactus cladodes promotes a feeling of satiety [41] [42], which reduces food intake thus promoting weight loss.
5. Conclusions
- It was concluded that prickly pear cactus cladode extracts from Kenya have potential to manage diabetes mellitus in humans in a dose- and time-dependent manner. The body weights of the animals fed on cactus cladode extracts declined suggesting that the cladodes could have played a role in controlling obesity, which is a major cause in the onset of type 2 diabetes mellitus. Thus, the present study provides insights on the potential application of prickly pear cactus cladodes from Kenya to manage diabetes mellitus.
ACKNOWLEDGEMENTS
- We acknowledge Kenya Forestry Research Institute (KEFRI), and Biotechnology Research Institute-KALRO for allowing us to use their laboratories and facilities to carry out this research and the Grand Challenges Canada Stars in Global Health (Grant number “S4 0297-01”) for the financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML