-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2016; 5(2): 21-25
doi:10.5923/j.diabetes.20160502.01

Oral Magnesium Potentiates Glutathione Activity in Experimental Diabetic Rats
A. O. Ige, E. O. Adewoye, E. O. Makinde
Applied and Environmental Physiology Unit, Department of Physiology, University of Ibadan, Ibadan, Nigeria
Correspondence to: A. O. Ige, Applied and Environmental Physiology Unit, Department of Physiology, University of Ibadan, Ibadan, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Antioxidants have been reported to prevent the development of diabetic complications. Oral magnesium treatment in diabetes has also been reported to potentiate the antioxidant defence system. This study investigated the glutathione antioxidant system (total glutathione, reduced glutathione, glutathione peroxidase) in experimental diabetic rats treated orally with magnesium. Wistar rats (n = 20) weighing 140 -160g were randomly divided into 4 groups of 5 animals each. Group 1 was control. Groups 2–4 were made diabetic with alloxan (120mg/kg i.p). Group 2 was diabetic untreated (DU), group 3 received magnesium (250mg/kg) orally (DMg250) while group 4 received insulin (3IU s.c) (DI) for 21days respectively. Blood glucose level was monitored using the glucose oxidase method throughout the duration of the study. At 21days post-treatment blood samples (7mls) were obtained from the retro-orbital sinus of each animal after light ether anaesthesia and analyzed for total glutathione, reduced glutathione and glutathione peroxidase activities respectively. Blood glucose level was increased (P<0.05) in the DU group compared to control, DMg250 and DI treated groups. Total glutathione values were decreased (P<0.05) in DU group (30.5±3.8) compared to control (54.2±10.9), DMg250 (45.7±9.7) and DI (36.8±4.0) treatment groups. Reduced glutathione values were significantly increased (P<0.05) in control (5.13±0.41), DMg250 (3.49±0.20) and DI (3.27±0.12) groups compared to DU (2.50±0.14). Glutathione peroxidase activity was significantly increased (P<0.05) in the DU (702.7±60.23), DMg250 (796.26±66.11), DI (896.0±43.88) treatment groups compared to control (421.22±64.35). Glutathione peroxidase values obtained in the DI treated group was significantly increased compared to DU. Oral magnesium treatment exerts hypoglycaemic effects and potentiates the glutathione antioxidant system in alloxan-induced diabetic rats.
Keywords: Diabetes mellitus, Magnesium, Antioxidants, Glutathione, Glutathione peroxidase
Cite this paper: A. O. Ige, E. O. Adewoye, E. O. Makinde, Oral Magnesium Potentiates Glutathione Activity in Experimental Diabetic Rats, International Journal of Diabetes Research, Vol. 5 No. 2, 2016, pp. 21-25. doi: 10.5923/j.diabetes.20160502.01.
Article Outline
1. Introduction
- Oxidative stress has been observed to play a significant role in the pathogenesis of diabetes mellitus and its complications [1]. In normal condition, the antioxidant defense system should minimize the levels of reactive oxygen species (ROS) produced during diabetes mellitus while allowing ROS useful roles of cell signaling and redox regulation [2]. Glutathione (GSH) has been described as a multifunctional intracellular antioxidant [3]. Chemically, it is a tripeptide compound consisting of 3 amino acids which are glutamic acid, cysteine and glycine [4]. It is an intracellular antioxidant known to maximize the activity of all the other antioxidants, including ascorbic acid, vitamins E and α-lipoic acid. The reduced form of GSH acts as a main intracellular antioxidative buffer with multifaceted action against tissue oxidative stressors [5]. The presence of the glutathione SH group during oxidative activity of ROS has been observed to cause the formation of glutathione free radical (GS-) or glutathione disulphide (GSSG-) which is also known as oxidized glutathione [4]. Glutathione has also been reported to be a substrate for glutathione peroxidase (GPx) – an enzyme which reduces hydrogen peroxide (H2O2) in the presence of superoxide dismutase [4]. Both, GPx and GSH have been observed to inhibit lipid peroxidation either directly or indirectly by attenuating lipid peroxides peroxidase [6]. The important function of GSH is thus the protection of cellular proteins against oxidative injury by ROS.Various micronutrients have been reported to exert antioxidant effects in diabetes mellitus [7]. These micronutrients include magnesium, zinc, iron, manganese, chromium and selenium and it has been suggested that some of the protective effects of these micronutrients may be related to their effects on the activity of the antioxidant enzyme glutathione peroxidase. Oral magnesium (Mg) intake has been reported to improve glycemic status, potentiate antioxidant production and inhibit or delay the onset of complications in diabetes mellitus [8]. It has also been suggested that magnesium deficiency may play a prominent role in the pathogenesis of diabetic complications [9]. Magnesium has also been reported to act as a precursor molecule for glutathione in the blood; it is required for the proper functioning of the enzyme gamma glutamyl transpeptidase (GGT) which is involved in the synthesis of glutathione [10]. This study investigated the effect of oral magnesium treatment on the glutathione antioxidant system (total glutathione, reduced glutathione, glutathione peroxidase) in alloxan-induced diabetic rats.
2. Materials and Method
2.1. Animals and Grouping
- Twenty male Wistar rats were obtained from the Central Animal House, College of Medicine, University of Ibadan. They were acclimatized in well aerated cages with alternating 12 hour light and dark cycles, maintained on standard rat chow and allowed free access to drinking water according to the regulations and ethics governing the use of animals’ in laboratory experiments of the University of Ibadan. They were randomly divided into 4 groups of 5 animals each. Group 1 was control. Groups 2 – 4 were made diabetic with a single intraperitoneal dose of alloxan monohydrate (120mg/kg). Animals with sustained blood glucose of ≥200 after 5days were considered as being diabetic [11]. Group 2 was diabetic untreated (DU); group 3 was diabetic treated orally with 250mg/kg magnesium (DMg250) while group 4 was diabetic treated subcutaneously with 3 IU/kg insulin (DI) for 21 days respectively. All treatments with magnesium were administered orally using oral cannula while insulin was daily prepared and administered subcutaneously at a dose of 3 IU/kg. Body weights and blood glucose levels were monitored before the induction of diabetes and on days 0, 7, 14 and 21 respectively after the establishment of diabetes mellitus using the glucose oxidase method [12].
2.2. Blood Collection and Biochemical Analysis
- At the end of the treatment period (21 days), blood samples (7ml) was obtained from the retro-orbital sinus of each animal under mild ether anaesthesia into a heparinised EDTA tubes to prevent coagulation. Four milliliters (4ml) of each blood sample was centrifuged at 1000g for 10mins at 4°C using a cold centrifuge to obtain plasma which was used for evaluation of the total glutathione and reduced glutathione levels. The remaining heparinised whole blood (3mls) was used for the determination of glutathione peroxidase activity. Total and reduced glutathione was assayed using the glutathione assay kit manufactured by RayBiotech Inc, USA. The kit employed a kinetic enzymatic recycling assay based on the oxidation of glutathione by 5, 5 – dithiobis (2 – nitrobenzoic acid) DTNB. The glutathione peroxidase (GPx) activity was assayed using the Glutathione peroxidase assay kit manufactured by Fortress diagnostics, United Kingdom. The kit utilized the oxidation of glutathione (GSH) by cumene hydroperoxide, a reaction catalyzed by GPx, as its assay principle.
2.3. Statistical Analysis
- The results obtained are expressed as mean ± SEM. Statistical significance was taken at P < 0.05 using the Student’s T-test. All analysis was carried out using the Microsoft excel 2007 statistical package.
3. Results
3.1. Body Weight Changes
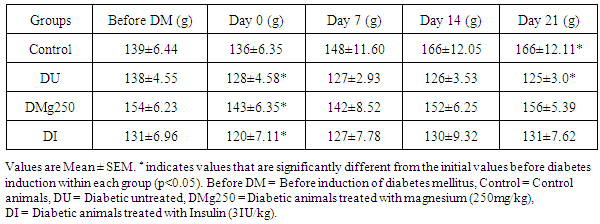
- Animals in the control group had a consistent increase in body weight with values at day 21 (166 ± 12.1g) being 19.4% higher than initial body weights (139 ± 6.4g) (Table 1). A consistent and significant reduction in body weight (P<0.05) was observed in the diabetic untreated group throughout the duration of the study. Average weight by day 21 (125 ± 3.5g) was 9.4% lower than the initial weight (138 ± 4.6g). Animals in the treatment groups (magnesium and insulin) had initial reduction in body weight after the establishment of diabetes mellitus (154 ± 6.2g vs. 143 ± 6.4g; 131 ± 7.0g vs. 120 ± 7.1g) however the weight of animals by day 21 in the treatment groups were similar to their initial values before induction of diabetes (156 ± 5.4g vs. 154 ± 6.2g; 131 ± 7.6g vs. 131 ± 7.0g) (Table 1).
|
3.2. Blood Glucose Evaluation
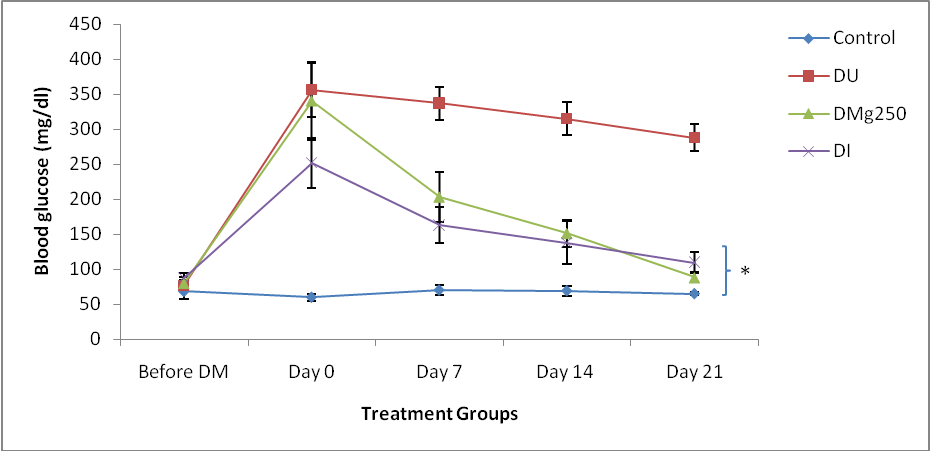
- Blood glucose level in the control animals was relatively stable throughout the duration of the study while an increase in blood glucose level was observed in the diabetic groups (DU, DMg250 and DI) on day 0 (356 ± 38.7 mg/dl, 340 ± 56.2 mg/dl, 252 ± 35.8 mg/dl) after the establishment of diabetes mellitus (fig. 1). Blood glucose level remained consistently high in the diabetic untreated group (DU) by days 7 (337 ± 23.5 mg/dl), 14 (315 ± 23.4 mg/dl) and 21 (288 ± 18.8 mg/dl) while values obtained in the magnesium and insulin treated groups were significantly reduced (P<0.05) on days 7 (203 ± 35.6 mg/dl; 163 ± 25.5 mg/dl), 14 (151 ± 19.1 mg/dl; 138 ± 30.6 mg/dl) and days 21 (88 ± 10.2 mg/dl; 110 ±14.4 mg/dl) compared to the DU group (fig. 1).
3.3. Total Glutathione, Reduced Glutathione and Glutathione Peroxidase Activity in Control, Magnesium Treated and Untreated Diabetic Animals
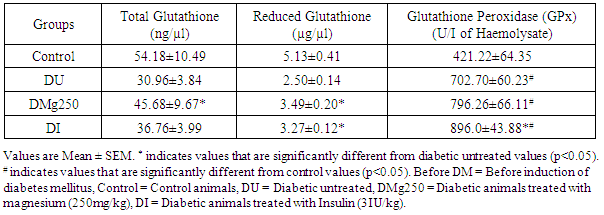
- The values obtained showed a 42.9% reduction (P<0.05) in total glutathione level in the diabetic untreated group (30.96 ± 3.84 ng/µl) (DU) compare to control (54.18 ± 10.49 ng/µl). Total glutathione values obtained in the DMg250 (45.68 ± 9.67 ng/µl) and DI (36.76 ± 3.99 ng/µl) treatment groups showed a 49.2% and 19.9% increase respectively compared to the DU group (Table 2). The DU group showed a significant reduction in reduced glutathione level (2.50 ± 0.14 µg/µl) compared to control (5.13 ± 0.41 µg/µl), DMg250 (3.49 ± 0.20 µg/µl) and DI (3.27 ± 0.12 µg/µl) treatment groups (Table 2). An increase (P<0.05) in glutathione peroxidase activity was observed in the DU (702.70 ± 60.23 U/I Haemolysate), DMg250 (796.26 ± 66.11 U/I Haemolysate) and DI (896.0 ± 43.88 U/I Haemolysate) treatment groups when compared to control values (412.22 ± 64.35 U/I Haemolysate). Glutathione peroxidase values obtained in the insulin treated animals (DI) were also significantly increased compared to diabetic untreated (DU) animals (Table 2).
|
4. Discussion
- Oxidative stress reflects an imbalance between the production of reactive oxygen species and antioxidant defence [13]. In diabetes mellitus, oxidative stress plays a pivotal role in the development and progression of diabetic complications [14]. Magnesium has been reported to exert hypoglycaemic effects in diabetes mellitus due to it glucose regulatory and stabilizing effects [15]. This was also observed in this study in the magnesium treated group with values obtained being comparable to those of the insulin treated animals (Figure 1). This suggests a possible similar hypoglycaemic mechanism between oral magnesium treatment and insulin treatment in diabetes mellitus. Furthermore magnesium has been reported to facilitate all insulin secretory and regulatory activities [8] and this may account for the insignificant difference in blood glucose level observed between the magnesium treated and insulin treated groups. The resultant glucose regulatory effect of magnesium and insulin could also be responsible for the recovery in body weight of these treatment groups (magnesium and insulin) compared to diabetic untreated animals that had a progressive and significant reduction in body weight considering their initial values (Table 1). In this study, an increase in total glutathione level in the magnesium treated animals was observed which suggests a potentiation of glutathione production (Table 2). Glutathione has been reported to be the major endogenous antioxidant produced by the cell [16]. It is known to participate in the neutralization of free radicals and reactive oxygen compounds as well as maintain exogenous antioxidants such as vitamins C and E in their active forms [17]. Glutathione exists intracellularly in two forms as both reduced glutathione and glutathione disulphide (GSSG). Reduced glutathione is the antioxidant form of glutathione. This study also shows an increase in reduced glutathione activity in the magnesium and insulin treated rats which also suggest a potentiating effect of magnesium and insulin on reduced glutathione production in these treatment groups. The similar values between the magnesium and insulin treatment groups may indicate possible similar glutathione potentiating mechanism in these treatment groups. Reduced glutathione has been reported to reduce protein free radicals produced by reactive oxygen species (ROS) to yield the glutathione free radical (GS-) which further reacts with GSH to form the free radical of glutathione disulphide (GSSG-). This donates an electron to the oxygen molecule, converting it to O2- which is then eliminated by superoxide dismutase [6, 18]. The observed glutathione potentiating effect of magnesium in this study is in accordance with the reports of Hsu et al., [19] who reported that magnesium is essential in the maintenance of GSH concentration in red blood cells thus protecting its membrane against oxidative damage. Furthermore, Mills et al. [20] has also reported that magnesium deficiency inhibits the biosynthesis of glutathione. Magnesium has been reported to be essential to the functions of the enzyme gamma glutamyl transpeptidase (GGT), an enzyme that plays an important role in the biosynthesis of glutathione by breaking down circulating extracellular glutathione thus releasing cysteine for intracellular re-assembly of glutathione [10]. This production of glutathione by the gamma-glutamyl cycle has been observed to be important in maintaining the glutathione level and the GSH: GSSG ratio [10]. Hence the observed increase in GSH activity (total and reduced glutathione) in the magnesium treated diabetic animals may possibly be due to its effect on the enzymes gamma glutamyl transpeptidase (GGT), glutamate cysteine ligase and glutathione synthetase respectively. Glutathione peroxidase (GPx) is an enzyme with peroxidase activity whose main biological role is to protect the cell from oxidative damage by reducing lipid hydroperoxides to their corresponding alcohols and reducing free hydrogen peroxide to water [21]. Magnesium has been reported to be directly involved in intracellular antioxidant defence mechanism by increasing the activity of the enzyme, glutathione peroxidase (GPx) [22]. This enzyme has been reported to increase the rate of reaction between glutathione and free radicals, particularly toxic hydrogen peroxide [22]. This study shows an increase in GPx enzyme activity in all diabetic animals (treated and untreated) compared to controls which is in accordance with the report of Maritim [23] who reported that GPx activity is increased in diabetes mellitus. The results obtained also show a further increase in glutathione peroxidase activity in the magnesium and insulin treated diabetic groups suggesting a potentiating effect of magnesium on the activity of this enzyme in these treatment groups (Table 2). This is in accordance with the report of Yavuz and Mollaoglu [22] who reported an increase in GPx activity in carbon monoxide toxic rats treated with magnesium.
5. Conclusions
- Oral magnesium treatment at 250mg/kg in diabetes mellitus exerts hypoglycaemic effects, potentiates glutathione production and maintains intracellular glutathione levels through glutathione enzyme pathways. It is therefore likely that in addition to the glucose regulatory effects of oral magnesium in diabetes mellitus, it may also potentiate the antioxidant defense mechanism through its effects on glutathione production and action.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML