-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2016; 5(1): 14-19
doi:10.5923/j.diabetes.20160501.03

High Intensity Interval Versus Continuous Moderate Aerobic Training as a Prophylaxsis of Diabetic Nephropathy
Mona Mohamed Taha1, Heba Ahmed Abdeen1, Rafeek Abdallah Abdelsamaia2
1Department of Cardiovascular/ Respiratory Disorder and Geriatrics, Faculty of Physical Therapy, Cairo University, Egypt
2Inorganic Chemistry Department, National Research Centre, Dokki, Cairo, Egypt
Correspondence to: Mona Mohamed Taha, Department of Cardiovascular/ Respiratory Disorder and Geriatrics, Faculty of Physical Therapy, Cairo University, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background and Purpose: The most common diabetic micro vascular complications is diabetic nephropathy. Exercise could ameliorate or prevent complications such as chronic kidney disease. High intensity interval training (HIIT) emerged as a new option of physical training to patients and health care professionals. This study aimed to evaluate and compare high intensity interval versus continuous moderate aerobic training as a prophylaxis of Diabetic nephropathy. Methods: Forty five sedentary male subjects, with type 2 diabetes; 40-55 years old were randomly allocated into three groups: High intensity interval training group I (HIIT; n=15) performed a high intensity interval training 3 times a week for 10 weeks, continuous moderate aerobic training group II (CMAT; n=15) received moderate continuous aerobic training 3 sessions per week for 10 weeks, and control group III (n=15) remain sedentary. Fasting blood glucose level and blood urea and creatinine were measured before the beginning and after the completion of the study. Results: At the end of the study; there were significant decreases in fasting blood glucose level and blood urea and creatinine in HIIT and CMAT groups. The percentage of improvement of fasting blood glucose level and blood urea and creatinine in the HIIT group were 14.53%, 9.53% and 28.26%, respectively; while those for the CMAT group were 9.89 %, 11.56% and 19.31% respectively. Between groups; there were significant differences in fasting blood glucose level, blood urea and creatinine in favor of group-I .Conclusions: Both HIIT and CMAT improved the glycemic control and renal function. HIIT could be more effective than CMAT in protecting from diabetic nephropathy.
Keywords: Interval training, Aerobic exercise, Nephropathy, Diabetes
Cite this paper: Mona Mohamed Taha, Heba Ahmed Abdeen, Rafeek Abdallah Abdelsamaia, High Intensity Interval Versus Continuous Moderate Aerobic Training as a Prophylaxsis of Diabetic Nephropathy, International Journal of Diabetes Research, Vol. 5 No. 1, 2016, pp. 14-19. doi: 10.5923/j.diabetes.20160501.03.
Article Outline
1. Introduction
- About 382 million people all over the world suffering from diabetes mellitus, expected to reach 592 million by 2035 [1]. This worldwide prevalence of diabetes is somewhat due to sedentary lifestyle and obesity. Patients with diabetic complications are increasing which will require a huge load on the healthcare system [2, 3].Patients differ significantly in their tendency to develop diabetes complications. It has been anticipated that there is a genetic basis for propensity or resistance to the development of diabetes complications to explain these observations, suggesting that factors other than glycaemic control may be significant in their pathogenesis [4].Hyperglycemia particularly in type 2 diabetes develops gradually and may be without symptoms so type 2 diabetes may be undiagnosed for many years and predisposes the patients to increased risk of microvascular and macrovascular complications [5].Kidney disease is considered the most unfavourable complication of diabetes, because of its considerable co-morbidity (blindness, need for dialysis, amputations, etc.), cost, and mortality (the annual mortality rate of diabetic patients with kidney failure on dialysis is about 25%) [6]. The major determinants of kidney disease and its progression to end-stage kidney failure in type 2 diabetes are uncontrolled blood glucose, blood pressure and albuminuria [7-9].The pathogenic factors of diabetic nephropathy could be due to low-grade inflammation. As hyperglycemia increased the production of reactive oxygen species which stimulates various pathways as formation of Advanced glycation end-products (AGEs), the polyol pathway production, hexosamine pathway and protein kinase C pathway which stimulate and propagate inflammatory processes, that leads to end-organ damage [10].Although Glomerular filtration rate (GFR) is considered the most important marker of kidney function, it cannot be easily measured in most clinical or research settings. Therefore estimating equations based on filtration markers such as serum creatinine and cystatin C are used. Other biomarkers such as albuminuria may precede kidney function decline and have demonstrated to have strong associations with disease progression and outcomes. Kidney function estimation was commonly made using serum creatinine concentration, blood urea nitrogen level and urine analysis [11].It has been shown that regular exercise with [12, 13] or without [12, 14, 15] dietary intervention and/or oral blood glucose-lowering medication [16, 17] have benefits in patients with Type 2 diabetes. However, nearly all of these studies were done in newly-diagnosed diabetes patients without complications and/or only insulin resistance [18].Physical activity has been prescribed for patients suffering from nephropathy although it is an important stage of renal insufficiency. The prescription is then influenced by the extent of renal insufficiency in addition to the presence of other associated diseases (anemia, hypertension, osteopathy etc.). Physical activity is essential in patients on dialysis respectively. After renal transplantation for maintaining peripheral muscle and its functions which not only improves the prognosis but also improves the quality of life and self-adequacy [19].It has been evidenced that moderate continuous aerobic exercise has favourable outcomes in diabetic patients, however recently High-intensity interval training has been proposed as more beneficial than aerobic exercise [20]. High-intensity interval training describes the physical exercise that is characterized by brief, intermittent bursts of strenuous activity, interspersed with rest periods or low-intensity exercise. Different physiological adaptations to HIT determined by numerous factors including the accurate nature of the exercise stimulus [21].So the aim of this study is to compare between the effect of high intensity interval exercise versus moderate continuous aerobic training as a prophylaxis for renal complications in the type 2 diabetic male patients.
2. Methods
2.1. Subjects
- Forty-five sedentary, volunteer male subjects, with established type 2 diabetes were recruited from Kaser El-Aini Hospital, Cairo-Egypt. Their ages ranged from 40 to 55 years. Inclusion criteria were: fasting blood glucose 140 <-mg/dl, body mass index (BMI) < 35 kg/m2. Exclusion criteria included the following: Diabetes duration < 5 years, BMI < 25 kg/m2, use of exogenous insulin, end-stage liver or kidney disease neuropathy, retinopathy, neuromuscular, cardiopulmonary, neuromuscular and/or psychological disease, other diseases contraindicated for physical activity.All patients were referred to the study by a physician. Randomization was performed simply by asking the patient to randomly choose a piece of paper which (I) or (II) or (III). So participants were randomly assigned into one of the three groups; high intensity interval training group (group- I; n=15) received high intensity interval training 3 sessions per week for 10 weeks or moderate continuous aerobic training group (group-II; n=15) received moderate continuous aerobic training 3 sessions per week for 10 weeks and control group (group-III; n=15) where subjects remain sedentary. The aim and procedures of the study were informed to eligible patients and signed a written informed consent and the study approved by an ethical Committee of the Faculty of Physical Therapy, Cairo University.
2.2. Evaluation
- All participants underwent an identical battery of tests. Demographic data including weight in kg, height in m, BMI (weight kg / height2) were all evaluated before the study. The main evaluated parameter was fasting blood glucose level and blood urea and creatinine which were performed before the beginning and after the completion of the study. To control the acute effects of exercise on hemodynamic and biochemical variables, all final testing was measured at least 24 to 36 hours after the last exercise session. The assessors were blinded to the participants’ treatment assignments. All subject’s data were collected using standard laboratory procedures.
2.3. Training
- Electronic treadmill (Vegamax, made in Taiwan) was used for training which consisted of walking/running on a treadmill 3 sessions/week for 10 weeks with a warm-up for 10 min at 50% of maximal heart rate (HRmax) and 5 min cool-down as the total exercise time was 40 min. High intensity interval training consisted of 4x4 min intervals at 80-85% of HRmax, with 3-min active recovery at 70% of HRmax between intervals whereas continuous moderate exercise training was walking at moderate intensity (50-70% of HRmax). Maximum heart rate was estimated according to the following formula: HRmax = 220 – age. A heart rate and the Borg scale of perceived exertion were monitored during the training to ensure that all subjects were exercising on their corresponding intensity of exercise. Continuous adjustment of the speed of the treadmill was used to avoid training adaptations to ensure training at the desired heart rate along the whole 10-week training program.
2.4. Statistical Analysis
- Statistical analysis was performed using SPSS software (version 16.0). Data were expressed as mean ± standard deviation (SD). Mean changes within groups (pre and post-study) were analyzed using Paired T-test while mean changes between groups (pre and post-study) were analyzed using one way analysis of variance (ANOVA) to test hypothesis within and between groups. The level of significance was set at p < 0.05.
3. Results
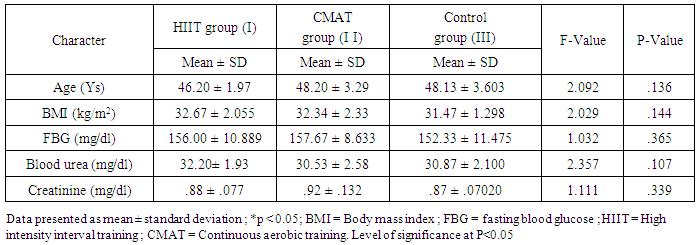
- A total of 45 diabetic male subjects participated in this study, including 15 in group I, 15 in group II and 15 in group III. Subjects in the group I adhered to the 10-weeks high intensity interval training program very well and Subjects in the group II adhered to the 10-weeks moderate continuous aerobic training program while subjects in the group III remain sedentary. Table 1 shows the baseline characteristics of the subjects at the beginning of the study. There were no significant differences in age, BMI, fasting blood glucose level, blood urea and creatinine among the three groups.
|
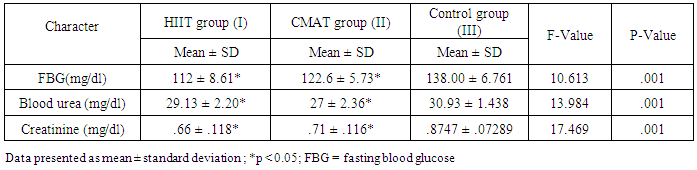
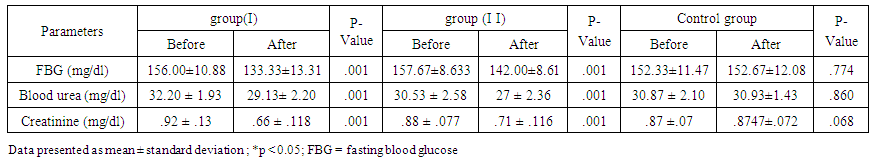 | Table 2. Physiological Characteristics of the Subjects before and after 10 Weeks |
|
4. Discussion and Conclusions
4.1. Discussion
- Recently, more attention has been focused on the beneficial effects of high intensity interval training (HIIT) programs to type 2 diabetes mellitus as a time-efficient training option. So, the aim of our study is evaluating the effects of exercise training of different frequencies and intensities in patients with T2DM. If research ultimately reveals that aerobic training especially HIIT could be protective of diabetic nephropathy, it will encourage adherence to aerobic training.In this study, both continuous moderate aerobic exercise and HIIT improved fasting blood glucose level and renal faction (creatinine and blood urea). But, HIIT shows a higher percentage of improvement concerning fasting blood glucose level and creatinine than continuous moderate aerobic training in type 2 diabetic patient.The results of improved blood glucose level in HIIT more than continuous moderate exercise have coincided with other previous studies, as Madsen et al., [22] stated that low volume HIIT for 8 weeks decreased fasting blood glucose concentration and enhanced glycaemic control. Moreover, Karstoft et al., [23] demonstrated that interval walking training preserves secretion of insulin and enhances insulin sensitivity when compared to matched continuous walking training energy expenditure. Additionally, Karstoft et al., [24] showed that in type 2 diabetes mellitus subjects, interval training improves glycaemic control free living and postprandial as compared to matched time-duration and oxygen-consumption matched continuous exercise. Boule et al., [25] stated that glycaemic control improved with high-intensity exercise greater than low-intensity exercise, and latest studies of interval training result in remarkable improvement on glycaemic control [26-28]. Recent work has shown that HIT improved insulin sensitivity and fasting glucose in previously sedentary, overweight subjects [29]. Exercise training improves peripheral insulin sensitivity may be due to increasing skeletal muscle glucose transport capacity, mediated in part by the protein GLUT4. Short-term HIT increased Skeletal muscle GLUT4 protein by nearly (2-fold) when compared to that noticed after high-volume endurance training [29]. In a pilot study on eight patients with type 2 diabetes, 2 weeks of low-volume HIT increased skeletal muscle GLUT4 content [27].The mechanisms mediating the improvement in glycemic control following HIT could be possible that the interval training (training with different intensities), increasing the peak exercise intensity, that theoretically has an advantageous for glycaemic control improvement [30], enhanced function of pancreatic β-cell [31], and increased overall fat oxidation and loss of abdominal fat when compared to continuous aerobic training [32].The United Kingdom Prospective Diabetes Study and the Diabetes Control and Complications Trial, reported that microalbuminuria risk significantly decreased by strict glycemic control [33]. Adolescents with type 1 diabetes had reduced exercise capacity, which was strongly associated with renal health [34].Our finding shows that renal function improved in both moderate continuous aerobic exercise and HIIT with a higher percentage of improvement in HIIT. .This result coincided with previous findings. Albright et al. suggested that the aerobic training protects the kidney from damage noticed in diabetic nephropathy [35]. Sikiru et al., [36] observed that moderate intensity aerobic training decreased creatinine production. So, it could protect and delays tissue damage. Additionally, aerobic training for 16 weeks at 60% of maximal aerobic capacity reduced renal fibrosis in spontaneously hypertensive rats by histological and molecular applications [37]. Tsutsumi et al., [38] also demonstrated that exercise seems to improve glomerular filtration rate and absorption from the uriniferous tubules, recover renal corpuscles shape in diabetic rats and could absorb and catabolise the advanced glycation end-products. Patrick et al., [39] demonstrated that HIIT significantly increases plasma albumin and decreases kidney weight and kidney weight-body weight ratio when compared to sedentary behaviour and low intensity aerobic exercise.Renal function improvement may be due to a reduction in the high-glucose levels toxic effect on the kidney [40]. As the Advanced glycation end-products (AGEs) are thought to be one of the main factors which lead to chronic diabetic complications [41, 42]. And the kidneys play a vital role in AGEs metabolism especially the proximal tubule cells which absorb and catabolize it through the glomerular filtrate. Its overload on kidneys is believed to be the reason of diabetic nephropathy [43]. Moreover, Insulin sensitivity improvement could inhibit the stimulation of nuclear factor kappa B and decreasing monocyte chemo attractant protein-1 expression, consequently reducing the kidney damage due to the inflammatory reaction [44, 45]. Additionally, the useful effects of aerobic training on oxidative stress induced by exercise [46] and decreased inflammatory reaction could also participate in this renal protection.
4.2. Conclusions and Recommendations
- Our study findings show that high intensity interval training could be used as an alternative option for traditional continuous aerobic exercise in improving glycemic control and protection of diabetic nephropathy.The limitations of this study are the small number of subjects and the sample wasn’t including females as they may be pre or post menopause in the study selected age group that may interfere with our results. Future researches are warranted to address this long-term effect of the interval training and the effect of different interval training protocols should be investigated. Also, additional studies are required to show the responses to interval training in different age population and samples including females and different grades of nephropathy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML