-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2016; 5(1): 7-13
doi:10.5923/j.diabetes.20160501.02

Does Obesity Increase the Prevalence of Chronic Kidney Disease in Caribbean Type-2 Diabetes Patients with or without Hypertension?
Chidum E. Ezenwaka1, Saleh Idris1, Gershwin Davis1, Lesley Roberts2
1Department of Para-Clinical Sciences, Faculty of Medical Sciences, The University of the West Indies, St Augustine Campus, Trinidad and Tobago
2National Organ Transplant Unit, Eric Williams Medical Science Complex, Mount Hope, Trinidad and Tobago
Correspondence to: Chidum E. Ezenwaka, Department of Para-Clinical Sciences, Faculty of Medical Sciences, The University of the West Indies, St Augustine Campus, Trinidad and Tobago.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Previous research studies have shown increased risk of renal disease in obese subjects without overt comorbidity factors. This study aimed to determine if obese diabetes patients have higher prevalence rates of different stages of chronic kidney disease (CKD) than their non-obese counterparts. Serum creatinine was measured in a cross section of 436 diabetes patients with or without hypertension after an overnight fast. Estimated glomerular filtration rate (e-GFR) was calculated using the CKD-EPI equation. The Kidney Disease Outcomes Quality Initiative classification was used to classify the different stages of CKD. Differences in CKD prevalence rates between the obese and non-obese patients were determined by chi-squared tests. Of the 436 patients, 88.8% and 42.9% had abdominal and generalized obesity respectively. A total of 142 (32.6%) patients had different stages of CKD; 107 (39.3%) diabetes patients with hypertension and 35 (21.3%) patients without hypertension. The prevalence of CKD stages 2, 3A, 3B, 4 or 5 were similar in obese and non-obese patients in both types of obesity (p > 0.05). Furthermore, irrespective of absence or presence of hypertension, there were no significant differences in the prevalence of CKD stages 2, 3A, 3B, 4 or 5 in obese and non-obese patients (p > 0.05). Obesity does not significantly increase the prevalence rates of different stages of CKD in Caribbean diabetes patients with or without hypertension.
Keywords: Abdominal obesity, Caribbean, Diabetes, Hypertension, Kidney disease, Obesity
Cite this paper: Chidum E. Ezenwaka, Saleh Idris, Gershwin Davis, Lesley Roberts, Does Obesity Increase the Prevalence of Chronic Kidney Disease in Caribbean Type-2 Diabetes Patients with or without Hypertension?, International Journal of Diabetes Research, Vol. 5 No. 1, 2016, pp. 7-13. doi: 10.5923/j.diabetes.20160501.02.
Article Outline
1. Introduction
- Several research reports have shown that obesity, both generalized and abdominal, is very prevalent among type 2 diabetes patients in the Caribbean [1-4]. Similarly, in the Caribbean, other research reports have shown that chronic kidney disease (CKD) is a major complication of diabetes [5-7]; to the extent that diabetes accounts for more than 28% of patients on renal replacement therapy [7]. Although a high percent of diabetes patients at the primary care settings in the Caribbean are usually overweight or obese [1-4], there are paucity of reports on the impact of obesity on the prevalence of CKD among diabetes patients in this population. Research studies in other populations have implicated obesity in renal function loss after nephrectomy [8], after renal transplant therapy [9-12] and in IgA nephritis [13]. However, the exact role of obesity in initiation of renal dysfunction has not been fully characterized because most studies were done in patients with concomitant renal risk factors such as diabetes and hypertension [14-19]. In this regard involvement of altered renal hemodynamics such as hyper-filtration has been reported in obese subjects [14, 16]. Furthermore, a study in non-obese healthy subjects showed that higher body mass index (BMI) is associated with higher filtration fraction (FF) calculated as the ratio of glomerular filtration rate (GFR) and effective renal plasma flow (ERPF) [20]. It has been argued that such unfavourable renal hemodynamic profile in the non-obese healthy subjects has the potential of increasing renal susceptibility in cases of patients with diabetes or hypertension [20]. Indeed clinical and experimental studies have shown increased risk of renal disease in obese subjects without overt comorbidity factors [8, 21-23]. Furthermore, there is epidemiologic data linking obesity and the metabolic syndrome to CKD [24]. Thus given that a previous study from this population reported high prevalence rates of the metabolic syndrome and its components including generalized and abdominal obesity [3], this study aimed to determine if diabetes patients with overt obesity have higher prevalence rates of different stages of CKD using estimated glomerular filtration rate classification of CKD [25, 26].
2. Materials and Methods
- Patient’s recruitment: A total of 436 type 2 diabetes patients (with and without hypertension, aged between 31 and 89 years) were recruited from adult Lifestyle Disease Clinics in two Regional Health Authorities (RHA) in Trinidad between September 2014 and March 2015. All patients gave informed voluntary consent to participate in the study. Patients were included in the study if they have been previously diagnosed with diabetes, consecutively visiting the clinic during the specified period and were on prescribed medication (commonly metformin, sulfonylureas, meglitinides, thiazolidinediones) for type 2 diabetes. None of the type 2 diabetes patients studied indicated use of insulin for management and none had type 1 diabetes. Patients with diagnosed hypertension and or on medication for treating high blood pressure or had measured blood pressure values greater than 135/85 mmHg [28]. Additionally, patients must be registered and visiting any of the adult Lifestyle Disease Clinics within the Regional Health Authority and must have had diabetes for at least one year prior to recruitment. Patients with cancer, infectious or inflammatory conditions, pregnant or those who declined voluntary consent were excluded from the study.
2.1. Study Protocol
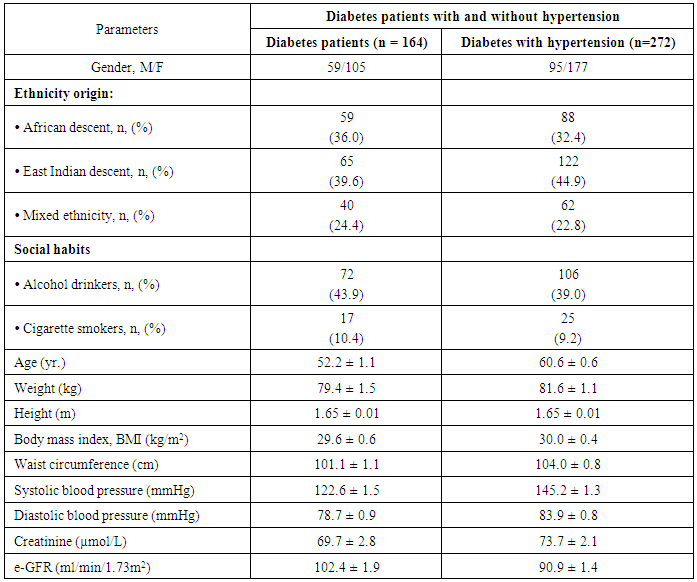
- 10 mL of blood was collected from each of the 436 patients after an overnight fast (10 – 12 hr) into red-top tubes. Serum were separated and stored frozen at -80°C until laboratory analysis. Anthropometric indices such as weight (measured in kg with clinic measuring scale), height (measured in meters with clinic measuring ruler) and waist circumference (at the level of the naval with the patient standing and breathing normally) and blood pressure (measured on the dominant arm in a sitting position using a standard semi-automated sphygmomanometer with adult cuff-size (Diammap, Pro-Care Auscultator 300, General Electric, USA) were measured. Other clinic information (age, gender, education, ethnicity) were obtained from each patient after a voluntary consent.
2.2. Ethical Consideration
- The Ethics Committees of the University of the West Indies and the Regional Health Authorities reviewed and approved the study protocol.
2.3. Laboratory Analysis
- Serum creatinine was measured in all the patients in our laboratory using dry slides with creatinine amidohydrolase and sarcosine oxidase enzymes in Vitros 4600 Multi-Channel Chemistry Auto-Analyser (Johnson & Johnson Ortho-Clinical Diagnostics Inc., Rochester NY, USA). The estimated glomerular filtration rate (e-GFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration calculator currently used in the Nephrology Unit of our hospital [27]:e-GFR = 141 × min (Scr /κ, 1)α × max(Scr /κ, 1)-1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black]where: Scr is serum creatinine in mg/dl, κ is 0.7 for females and 0.9 for males, α is -0.329 for females and -0.411 for males, min indicates the minimum of Scr /κ or 1, and max indicates the maximum of Scr /κ or 1.
2.4. Definitions
- i. Generalized and abdominal obesity: Generalized obesity was defined in this study as body mass index (BMI)> 30kg/m2 while abdominal obesity was defined as waist circumference ≥ 80cm (f) or 94cm (M) based on International Diabetes Federation (IDF) definition for the metabolic syndrome [28]ii. Chronic kidney disease (CKD) stages The different stages of CKD was calculated based on the Kidney Disease Outcomes Quality Initiative (KDOQI) classification [25, 26]:- Ÿ Stage 1: GFR > 90 ml/min/1.73m2 - normal kidney function Ÿ Stage 2: GFR = 60-89 ml/min/1.73m2 - Mildly reduced kidney function Ÿ Stage 3A: GFR = 45-59 ml/min/1.73m2 -moderately reduced kidney function Ÿ Stage 3B: GFR = 30-44 ml/min/1.73m2 - Moderately reduced kidney function Ÿ Stage 4: GFR = 15-29 ml/min/1.73m2 - severely reduced kidney function Ÿ Stage 5: GFR < 15 ml/min/1.73m2 or on dialysis - very severe or End stage kidney failure
2.5. Statistics
- The statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS). Chi-squared (X2) test was employed for all categorical variables in determining the differences between the obese and non-obese group of patients. A p-value less than 0.05 was considered significant.
3. Results
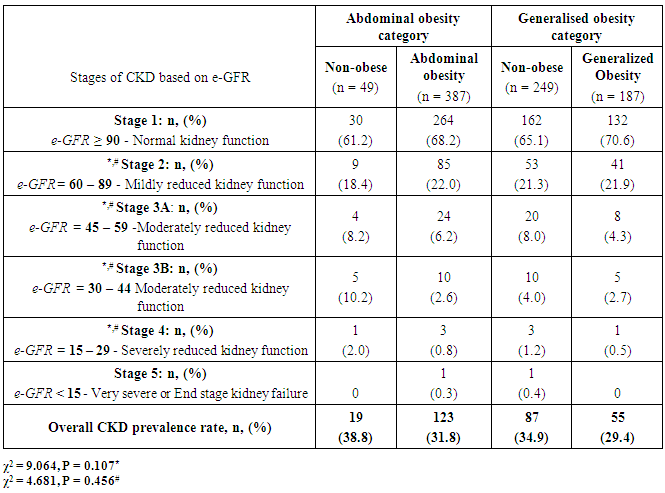
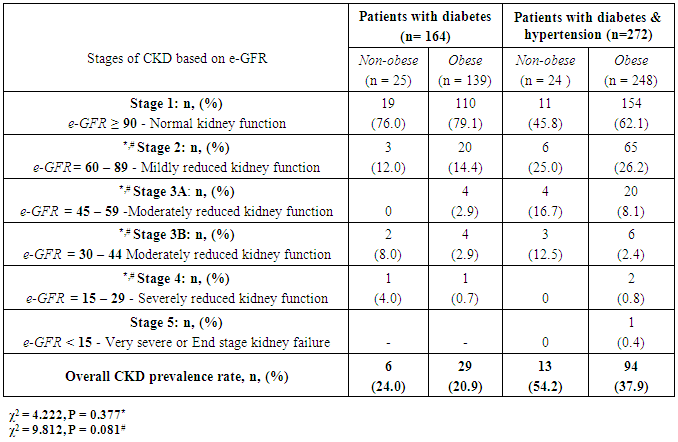
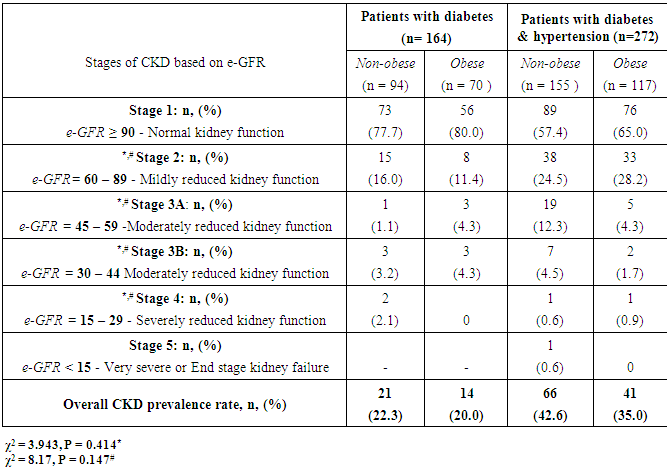
- The results of quantitative parameters are expressed as mean ± SEM and categorical data are expressed in percentages (%). Table I shows the socio-demographics, age, anthropometric indices, blood pressure and estimated glomerular filtration rate (e-GFR) of the patients studied. Of the 436 patients studied, 387 (88.8%) and 87 (42.9%) had abdominal and generalized obesity respectively (Table 2). Fig. 1 shows that diabetes patients with hypertension had higher percentages of abdominal and generalized obesity compared with diabetes patients without hypertension. Of the 436 diabetes patients studied, a total of 142 (32.6%) patients had different stages of CKD; distributed as 107 (39.3%) of diabetes patients with hypertension and 35 (21.3%) of diabetes patients without hypertension (Tables 3 & 4). The prevalence of CKD stages 2, 3A, 3B, 4 or 5 were similar in obese and non-obese patients irrespective of the type of obesity (p > 0.05, Table 2). Furthermore, analysis for the effect of abdominal obesity showed that there were no significant differences in the prevalence of CKD stages 2, 3A, 3B, 4 or 5 between abdominally obese and non-obese diabetes patients with or without hypertension (p > 0.05, Table 3). Similar analysis for generalized obesity showed that the prevalence rates of CKD stages 2, 3A, 3B, 4 or 5 between the generalized obese and non-obese diabetes patients were similar with or without hypertension (p > 0.05, Table 4).
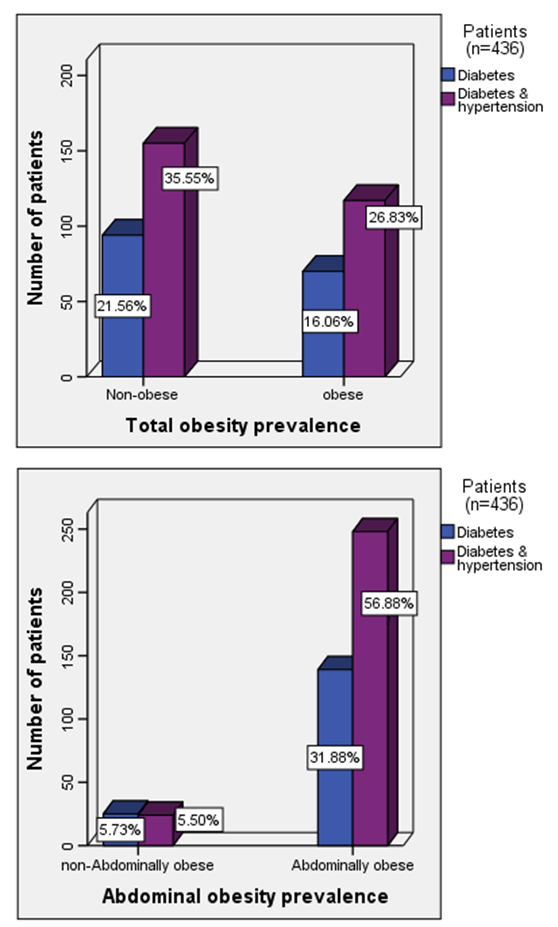 | Figure 1. Prevalence rates of obesity in diabetes patients with and without hypertension |
|
|
|
|
4. Discussion
- The results of this study showed i. high prevalence rates of abdominal (88.8%) and generalized (42.9%) obesity amongst the diabetes patients studied,ii. that diabetes patients with hypertension had higher prevalence rates of generalized (26.8%) and abdominal (56.9%) obesity than patients without hypertension, iii. high prevalence rate (32.6%) of overall CKD which was predominant in patients with hypertension, andiv. that the prevalence of CKD stages 2, 3A, 3B, 4 or 5 were similar in obese (abdominal and generalized) and non-obese diabetes patients with or without hypertension.The finding of high prevalence rate of generalised and abdominal obesity in this population is not entirely surprising because it is consistent with previous reports of high prevalence rates of obesity in this population [1-4]. Although we employed the International Diabetes Federation obesity cut-off point for the definition of the metabolic syndrome [28], another previous study in the same population that used a higher cut-off points for obesity reported similar high prevalence rates [2]. Of greater concern was the finding that diabetes patients with hypertension had higher prevalence rates of generalised and abdominal obesity than diabetes patients without hypertension. The basis of this differential is not completely understood but can be explained from epidemiological perspective. For instance, epidemiological report shows that when diabetes is prevalent in obese individuals, it varies according to gender and ethnicity [29] whereas hypertension and morbid obesity coexist in 50 to 70% of cases [30].The finding of a high prevalence rate of overall CKD among the patients studied is also consistent with previous reports of the burden of CKD among diabetes patients in this population [5-7], and the observation that diabetes patients with hypertension were more affected has been previously reported [31]. Of greater interest however was the finding that the prevalence rates of the CKD stages 2, 3A, 3B, 4 or 5 were similar in obese (abdominal and generalized) and non-obese diabetes patients with or without hypertension given that obesity is a major comorbidity factor for diabetes in this population [1-4] and CKD a common complication of diabetes [3, 6, 7, 31]. Of further interest was the observation that the obese (abdominal and generalized) patients tended, albeit insignificant, to have higher percentage of their numbers with normal kidney function (e-GFR > 90 ml/min/1.73m2) than non-obese patients. A previous study in non-obese healthy subjects have demonstrated that higher body mass index (BMI) was associated with higher filtration fraction (a ratio of glomerular filtration rate and effective renal plasma flow) [20]. The authors however cautioned that the observation has the potential of enhancing unfavourable renal hemodynamic profile in patients with diabetes or hypertension [20]. Although the exact mechanistic role linking obesity to CKD has not been completely characterized [14-19], several research reports have implicated obesity in renal dysfunction after unilateral nephrectomy and after renal transplant therapy [8-12]. Additionally, clinical and experimental studies have also shown increased risk of renal disease in obese subjects without overt comorbidity factors [21-23]. Although the present study did not significantly show that obese patients have higher prevalence of CKD than non-obese patients, virtual inspection of the trend of the data, shows that more patients with CKD were included in abdominally obese category than in generalized obesity group (Table 2). Indeed abdominal obesity has been shown to be a better predictor of hypertension, dyslipidemia and the metabolic syndrome than generalized obesity [32], and abdominal obesity is central to the metabolic syndrome [28]. It has been suggested that obesity and the metabolic syndrome are strong independent risk factors for CKD and end-stage renal disease and may have mechanistic links to CKD [24]. Thus given a previous report of increased prevalence of the metabolic syndrome and its components in diabetes patients in this population [3], the risk and prevalence of CKD amongst the patients may be intrinsically woven in abdominal obesity, diabetes, hypertension, insulin resistance, hemodynamic factors and metabolic syndrome [24]. The results of the study should be interpreted with caution on the basis of some limitations. First, it is possible that when the patients were categorised on the basis of obesity the sample size lost the statistical power to detect differences in the prevalence rates of different CKD stages between the obese and non-obese patients. Secondly, the obesity classification cut-off points used in the study did not take into account the multi-ethnic configuration of the study population that has many patients of South-east Asia descent; an ethnic group that requires a different body habitus categorisation criteria [28].
5. Conclusions
- It is concluded that obesity (abdominal or generalized) does not significantly increase the prevalence rates of different stages of CKD in diabetes patients with or without hypertension. We recommend further studies in this and other populations with enlarged sample size.
ACKNOWLEDGEMENTS
- This study was supported with a research grant from the University of the West Indies, St Augustine Campus. Mr. Saleh Idris was on a Pre-doctoral Fellowship from Bayero University, Nigeria. We thank all the patients for their blood, Ms Angela for technical assistance, the Regional Health Authorities for permission to conduct the research and the Nurses at the Lifestyle Disease Clinics of the Regional Health Authorities.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML