-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2015; 4(4): 67-72
doi:10.5923/j.diabetes.20150404.01
Effect of Intermittent Pneumatic Compression Therapy on Healing of Diabetic Foot Ulcer
Fatma A. Mohamed1, Heba A. Bahey El-Deen2
1Faculty of Physical Therapy, Cairo University, Giza, Egypt
2Faculty of Physical Therapy, Misr University for Science and Technology, Giza, Egypt
Correspondence to: Fatma A. Mohamed, Faculty of Physical Therapy, Cairo University, Giza, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Background: Foot ulcer is the most common complication that leads to amputation in diabetic patients. Purpose: To study the effect of intermittent pneumatic compression therapy (IPCT) in enhancing healing of chronic diabetic foot ulcer. Methods: Thirty-eight patients (13 men & 25 women) with type II diabetes and foot ulcer grade ІІ according to Wagner classification were recruited to participate in this study. They were classified into two groups; study group consisted of 18 patients (9 men and 9 women) and control group consisted of 20 patients (4 men and 16 women). The mean values of their ages were 53.83±2.50 years and 56.60±3.40 years respectively. Both groups received standard medical wound care for diabetic foot ulcer, in addition, the study group received a sequential IPCT for 120 minutes, three times/week for 8 weeks at inflation pressure 70 mmHg. Ulcer surface area was measured in squared centimeter by photographing it and using ImageJ software before and after the study period. Results: The results of this study revealed statistically significant improvement in wound surface area measurement in patients of the study group while non-significant change was observed in patients of the control group. Conclusions: IPCT is a useful adjunct to the standard medical wound care of chronic diabetic foot ulcer to enhance healing and avoid long term complications.
Keywords: Diabetic foot ulcer, Intermittent Pneumatic Compression Therapy (IPCT), Wound surface area (WSA) measurement
Cite this paper: Fatma A. Mohamed, Heba A. Bahey El-Deen, Effect of Intermittent Pneumatic Compression Therapy on Healing of Diabetic Foot Ulcer, International Journal of Diabetes Research, Vol. 4 No. 4, 2015, pp. 67-72. doi: 10.5923/j.diabetes.20150404.01.
Article Outline
1. Introduction
- Diabetes mellitus is a growing health problem all over the world. It is expected that Egypt will be ranked as one of the top 10 countries having the highest percentage of diabetic patients in 2030 [1]. The most common and disabling complication in diabetic patients is foot ulcer [2]. Diabetic patients have up to 25% chance throughout the life to develop foot ulcer with major increase in the percentage of subsequent unhealed infection that requires amputation [3]. Diabetic foot ulcer results from simultaneous action of multiple contributing causes [4]. First, peripheral neuropathy has the most important role as it decreases the protective sensations of pressure and pain and leads to atrophy of the small foot muscles which consequently alters the pressure distribution on the foot bones leading to foot deformities and ulceration [4, 5]. Second, peripheral arterial disease of the lower limb causes ischemia and impaired healing of the ulcer [6]. Impaired quality of life and high burden for both patients and society are associated with diabetic foot ulcers [5]. Intermittent pneumatic compression (IPC) is an application of alternating compressing pressures at a controllable rate on the lower limb [7]. It is used as an effective intervention strategy for different circulatory diseases [8]. Previous clinical studies demonstrated its efficacy in accelerating the venous ulcers healing [9], preventing deep venous thrombosis [10], lymphedema treatment [11] and increasing arterial flow to distal limbs so improving the arterial ischemia [12].Finding out modalities to enhance healing of diabetic foot ulcers is essential in order to avoid the related complications including amputation. Therefore, this study was designed to investigate the effects of intermittent pneumatic compression therapy for enhancing healing of diabetic foot ulcers.
2. Methods and Materials
- SubjectThe effects of pneumatic compression therapy on the healing of chronic diabetic foot ulcers were studied on forty patients with type II diabetes mellitus from both sexes (13 men & 27 women) with grade ІІ foot ulcer (ulcer extension to ligament, tendon, joint capsule, or deep fascia without abscess or osteomyelitis) according to Wagner classification [13]. Their ages ranged from 50 to 60 years and all were recruited from the outpatient clinic of National Institute of Diabetes, Cairo, Egypt. Patients were randomly allocated into two groups using one to one base; study group consisted of 20 patients (9 men and 11 women) received IPCT in addition to the standard medical wound care for diabetic foot ulcer and control group consisted of 20 patients (4 men and 16 women) received the standard medical wound care for the diabetic foot ulcer only. Both groups were managed concurrently. After selection of patients, all of them signed a consent form that they agreed to participate in the study.Inclusion Criteria Patients suffering from type II diabetes mellitus for minimum of five years with unilateral foot ulcer grade ІІ according to Wagner classification, nonsmokers and being co-operative were included in the study. All patients participated in this study were receiving their physician-prescribed medications regularly.Exclusion CriteriaPatients were excluded from the study if they had one of the following criteria: ● Paralysis of the affected leg, ● Receiving other physical therapy approaches for foot ulcers. ● Congestive heart failure or with cardiac pacemaker.● Severe wound infection or calf wounds.● History of leg fractures or metal implants in the affected leg. ● Patients with history of deep venous thrombosis or varicose vein surgery.Withdrawal Criteria Patients with deep vein thrombosis sustained ≤ 6 months before the study period or during the course of the study and those who cannot tolerate compression therapy were withdrawn from the study.This study was reviewed and was approved by the Ethics Committee of Faculty of Physical Therapy, Cairo University and was conducted from October 2013 to November 2014.In the initial sessionThe study protocol, its objectives and demonstration on equipment and procedures were explained in detail to all the patients before the initial assessment. Complete medical history was collected in the first session, physical examination was performed by a physician for all patients with particular attention paid to identify any long-term complications of diabetes.Instrumentations and Procedures for EvaluationEach ulcer was photographed by a digital camera. A metric ruler was located beside the wound in the image frame. Wound surface area (WSA) measurement was carried out using Image J software. Joint Picture Expert Group (JPG) was the image format loaded in Image J 1.46r software. A straight line of 0.5 cm was drawn along the metric ruler scale in the image using a straight line drawing tool., A “Set Scale” function was then chosen from Analyze pull-down menu then the length value of the drawn line (0.5 cm) in the field of “Known Distance” was entered. The Image J software now maps the measured digital distance in pixels to the 0.5 cm given distance (pixels to Cm scale) and calibrate the measuring tools for the planned measurement. By using the freehand drawing tool from the tool bar, the perimeter of the wound image was traced. To measure WSA, the Measure function was selected. The WSA was calculated instantaneously in square centimeters [14].Each measurement was obtained by the same evaluator before starting of the study. It was repeated after 24 sessions (three sessions per week for eight weeks) of IPCT in patients of the study group and after two months in patients of the control group.Instrumentations and Procedures for TreatmentPneumatic compression device (DL2002B Basic, Korea) was used for application of treatment in the study group. It consisted of a pneumatic impulse generator with dimensions of 190 x 250 x 210 mm and 2.8 Kg weight providing a pressure range of 20-240 (+/- 20) mmHg and a large foot, leg and thigh cuffs. Large-bore elastic tube connects the compression device to the cuff. The pump throughout the study was set to be operated at a maximum inflation pressure of 70 mmHg and deflation pressure of 0 mmHg that is provided sequentially for 120 min. with inflation time equals 4 seconds, and deflation time equals 16 seconds, with 3 cycles/min. Intermittent pneumatic compression was applied 2 hours/day, three times per week for 8 weeks with a total of 24 sessions. A regular inspection of the IPC device was done in order to ensure the optimal efficiency of the device through the duration of each session.The Typical Treatment Session with IPC Device Was Given as the Following SequencesEach patient was instructed to wear light clothes and placed in a relaxed recumbent position for 5 minutes before the application of the first compression cycle for ensuring accommodation of the peripheral blood flow. The ulcer was covered with thin plastic wrap to prevent contamination. The patient’s lower limb was placed within the deflated cuff of the IPC device, and the patient was positioned in a semi erect position. The four connectors of the tube were attached to the four leg cuff segments and the other united connectors were attached to the pump unit. Throughout the study, the pump was set to be operated at a maximum inflation pressure of 70 mm Hg, delivered sequentially for 120 minutes. Any discomfort induced by the application of the pump was graded as none, mild, moderate, and severe. In cases of severe discomfort, the pump was discontinued and the patient was excluded from the study. The device was switched off automatically after 30 minutes and it was reset again to complete the total session duration of 120 minutes. After completing 120 minutes of treatment the device was switched off automatically. The connectors were removed from both sides and the patient was assisted to take off the foot leg thigh cuff.The Standard Medical Wound Care for the Diabetic Foot UlcerAll patients in both groups have received the standard medical wound care for the diabetic foot ulcer that consisted of dressing with topical antimicrobials, debridement and offloading by wearing special foot wear [6].Statistical AnalysisData were collected before and after the study period (eight weeks) for the two groups. The data collection was taken with the same sequence and procedures for patients of both groups. Paired "t" test was used to determine the significance of difference in the mean values between the observed results in the initial and the final evaluations of wound surface area measurement within each group. Independent "t" test was used to compare the significance of difference in the mean value of wound surface area measurement between the initial and the final evaluations between groups. The level of significance was considered when p ≤ 0.05.
3. Results
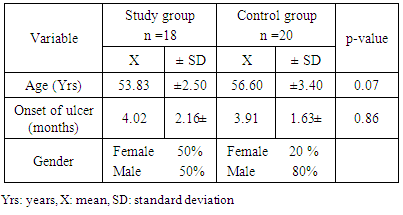
- Forty patients fulfilled the inclusion criteria of the study. Two patients (women) were withdrawn from the study group due to their inability to tolerate the compression therapy. The patients were randomly assigned into study and control groups comprising of 18 patients (9 men and 9 women) and 20 patients (4 men and 16 women) respectively. The mean values of their ages were (53.83±2.50) & (56.60 ±3.40) years for the study and control groups respectively with no significant difference between both groups (P = 0.07). The presented results in Table 1. showed non-significant differences in the pretreatment mean values between the two groups regarding the onset duration of the ulcer.
|
|
4. Discussion, Conclusions and Recommendations
4.1. Discussion
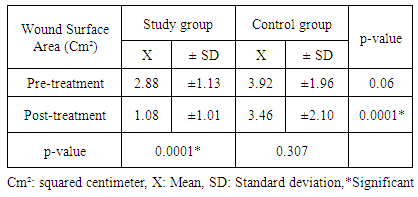
- Twenty five percent of diabetic populations with peripheral neuropathy and peripheral arterial disease (PAD) are more vulnerable to foot ulcer which may lead to diabetes-induced lower limb amputations [15, 16]. Intermittent pneumatic compression (IPC) is a well-documented safe and effective, noninvasive method where a device exerting an external dynamic pressure is used to treat peripheral vascular disease and chronic wounds, as well as prevention of deep venous thrombosis [9, 10]. This study was conducted to determine the effect of intermittent pneumatic compression therapy on healing of chronic diabetic foot ulcers. Our study presented the already known concept of using the mechanical effect of exerting sequential external venous pressure to improve peripheral arterial blood flow and its collaterals. Consequently, this would be clearly reflected on healing of chronic diabetic foot ulcers. The results of the present study proved a significant improvement of the ulcer surface area after IPCT. Patients with history of deep venous thrombosis (DVT) were excluded from this study as Malone et al., [17] reported that the capability of venous system to respond to compression decreases in patients who suffered from DVT as the compliance and elasticity of veins changed post thrombotic event. A study conducted by Eze et al., [18] proving that lower limb elevation improves the hemodynamics of the compression therapy. However, the current study did not follow the same trial opting for the semi-erect position [19] with extended lower limbs for all participants during the conduction of IPC treatment session. Since it was experimentally proved that it is the most comfortable position by all patients rather than elevated lower limbs position 45 degrees provided that each session lasts for 120 minutes. Furthermore, Lurie et al., [20] concluded that IPCT by using pump device was more efficiently increasing the venous flow of the popliteal vein compared to the elevation of the lower limbs alone. Additionally, Malone et al., proved [17] in their study that maximal velocities of venous blood flow can be achieved for extended period of time and remains constant after 120 minutes of IPCT application. The present study utilized sequential pressure applied on foot, leg and thigh consecutively which acts as wavelike compressor that leads to an increase of the blood flow velocity by over 200% within the vascular lumen [12, 20, 21, 22]. On applying IPC to the lower limb, a pulsatile flow caused by the sudden local pressure, leads to pumping the blood forward resulting in distention of the venous lumen. This distention exerts up to 20% of compressive strain on the venous endothelial cells, while the increased flow velocity imposes an increased shear stress on these same endothelial cells. This creates a state of arterio-venous pressure gradient causing a reduction in the venous pressure with corresponding increase in the arterial pressure and its flow velocity [23-25].This higher level of flow velocity increases the shear stress within the arterial endothelial inner layer. This shear stress has been found to increase the production of many humoral substances as prostacyclin and tissue plasminogen activator enhancing endogenous fibrinolytic activity and tissue factor pathway inhibitor that reduces coagulation effect [26-30], as well as a release of local vasodilator such as nitric oxide that inhibit platelet aggregation and monocyte adhesion to endothelial surfaces [9, 31].Moreover, Chen et al., [25] and Comerota, [9] stated that IPCT increases tissue perfusion resulting in improved oxygen diffusion and transcutaneous oxygen tension due to decreased interstitial fluid accumulation and venous stasis. All these previous effects were confirmed by the results of the present study and explained what was achieved by the significant improvement of chronic diabetic foot ulcer healing reflected by ultimate reduction of ulcer surface area. Where the mean values of ulcer surface area measurement in the study group was (1.08 ±1.01 Cm²) when compared with the pretreatment mean values which was (2.88 ±1.13 Cm²) (P value 0.0001) and the corresponding mean values of the control group was (3.46 ±2.10 Cm²) when compared with the pretreatment mean values which was (3.92 ±1.96 Cm²) (P value 0.307). The mean difference between the mean values of the study group that received IPCT in addition to standard medical treatment and the control group that received standard medical treatment only post 8 weeks was 2.37 with P value 0.0001. Significant increase of popliteal arterial blood flow was confirmed when using combined foot calf IPCT simultaneously. This improvement was reflected by causing a good relief of resting pain in patients who suffered from peripheral arterial disease in the lower limbs [32]. On the other hand, the current study preferred the sequential application of gradient hold-release action of IPCT that creates wave like pulse representing the dynamic compression of muscular pump to promote microcirculation and assist wound healing [16]. These results were demanded to ultimately enhance healing of diabetic ulcer. Many studies also added that a sequential compression device would increase venous return and preload volume that improves peripheral arterial blood flow especially in the popliteal artery [33, 34]. Jude et al., [35] stated that in diabetic patients with underlying ischemia, the distribution of peripheral arterial disease is greater in the arterial tree below the knee where the popliteal artery is located. Based on the previous evidences, our study relied on IPCT which augments peripheral blood flow in the popliteal artery. Our study utilized inflation pressure equal to 70 mmHg with 4 seconds inflation time and deflation pressure equal to 0 mmHg with 16 seconds deflation time repeated 3 cycles/min. The inflation provided compressions on four regions in the affected lower limb in a sequence. Those parameters coincided with Comerota [9], who reported that application of external pressure of 50 mmHg is enough to cause forward acceleration of the blood flow. Additionally, the results contradicted with other studies which proved that application of high-pressure devices at 120 mmHg have been shown to increase lower extremity skin perfusion associated with improved collateral circulation. Furthermore, they suggested that a larger volume of blood would be compressed with high-pressure, rapid-inflation compression [18, 36, 37]. Although previous studies proved that 120 mmHg inflation pressure had a superior effect. Moreover, compression of deep veins needs high pressure pump [38]. The present study opted for 70 mmHg pressure to avoid compromised peripheral arterial perfusion and sensory complications that diabetic foot ulcer patients suffered from. Moreover, slow inflation was utilized in this study since rapid inflation deprives IPCT action from its sequentiality.
4.2. Conclusions and Recommendations
- From the obtained results, it can be concluded that sequential IPCT three times/week for 8 weeks at an inflation pressure 70 mmHg would enhance the healing of chronic diabetic foot ulcer. The results also supported the known theory that ascertained the effect of IPCT in augmenting the peripheral blood flow and its velocity through widening of the arterio-venous pressure gradient and release of nitric oxide with its vasodilator effect. The results of the current study recommend the use of IPCT as a safe adjunct to the standard medical wound care of diabetic foot ulcer to enhance the healing and avoid the long term complications including lower limb amputation. Further future researches are recommended to investigate the effects of IPCT on deeper foot ulcers and for longer duration than 8 weeks to investigate the time needed for full ulcer closure.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML