-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2015; 4(3): 58-65
doi:10.5923/j.diabetes.20150403.03

Iron Deficiency in Non-dialyzed Patients with Type 2 Diabetes: A Cross-Sectional Study
Silvia Stefania Iancu 1, Cosmina Ioana Bondor 2, Mariana Coca 3, Rodica Rahaian 3, Ioan Andrei Veresiu 1, 4
1Center for Diabetes, Nutrition and Metabolic Diseases, Cluj-Napoca, Romania
2Department of Medical Informatics and Biostatistics, Iuliu Haţieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania
3Laboratory Department, County Emergency Hospital Cluj, Romania
4Department of Diabetes, Nutrition and Metabolic Diseases Iuliu Haţieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania
Correspondence to: Silvia Stefania Iancu , Center for Diabetes, Nutrition and Metabolic Diseases, Cluj-Napoca, Romania.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background. Iron deficiency (ID) is a major cause of anemia in diabetes. Data are sparse regarding iron metabolism and its association with anemia in non-dialyzed patients with diabetes and without other overt causes for low haemoglobin level. The objective of this study was to determine the prevalence and characteristics of ID in non-dialysis dependent patients with type 2 diabetes and to assess its association with anemia and diabetic complications. Methods. Data from 400 non-dialysis dependent patients with type 2 diabetes (≥2 years) (excluding patients treated with iron therapy or erythropoietin-stimulating agents, or with foot lesions or amputations or other known clinical causes of ID) were analyzed in a cross-sectional, single-center study. ID was defined as ferritin <100 ng/mL (absolute ID), or ferritin ≥100 ng/mL and transferrin saturation (TSAT) < 20% (functional ID), and anemia as hemoglobin <13.5 g/dL in men ≤70 years, 12.0 g/dL in men > 70 years and <11.5 g/dL in women. Results. 315 patients (78.8%) were iron deficient (186 absolute ID, 129 functional ID). ID anemia (IDA) was present in 74 patients (18.5%) of 89 patients with anemia from any cause (22.3%). Female gender was the only independent predictor of absolute ID (OR=2.30 95%CI 1.44-3.38, p<0.001). Increasing C-reactive protein was predictive of functional ID (OR=1.03 95%CI 1.01-1.05, p=0.001). The prevalence of IDA and all-cause anemia increased significantly with severity of chronic kidney disease (CKD) and urinary protein excretion (both p<0.001). Concerning diabetes complications we found that severe CKD was significantly associated with IDA (OR 5.32, 95% CI 2.52-11.25; p<0.001) and anemia of any cause (OR 8.39, 95% CI 3.87-18.23; p<0.001); autonomic neuropathy was significantly associated with absolute ID (OR=2.18, 95%CI 1.08-4.43, p=0.027) and proliferative retinopathy was significantly associated with functional ID (OR=1.75, 95% CI 1.02-3-02, p=0.041). Conclusions. Iron metabolism abnormalities, as determined by metabolic inflammatory status and the presence of ID, are frequent in type 2 diabetes and increase with severity of CKD, particularly in patients with autonomic neuropathy and proliferative retinopathy.
Keywords: Anemia, Chronic kidney disease, CKD, Diabetes, Ferritin, Iron, Iron deficiency, Neuropathy, Transferrin
Cite this paper: Silvia Stefania Iancu , Cosmina Ioana Bondor , Mariana Coca , Rodica Rahaian , Ioan Andrei Veresiu , Iron Deficiency in Non-dialyzed Patients with Type 2 Diabetes: A Cross-Sectional Study, International Journal of Diabetes Research, Vol. 4 No. 3, 2015, pp. 58-65. doi: 10.5923/j.diabetes.20150403.03.
Article Outline
1. Introduction
- Anemia is frequently associated with diabetes, affecting up to one in five patients [1, 2]. The presence of anemia in patients with diabetes is associated with a significantly increased risk of cardiovascular events [3, 4]. A hyperglycemic environment in renal interstitium induces hypoxia and promotes progression of renal disease [5], which in turns impairs erythropoietin production [6], such that mean hemoglobin (Hb) is lower than in individuals without diabetes [7]. Loss of renal function due to diabetic nephropathy can also make a major contribution to anemia though blunting of erythropoietin synthesis [8]. Even in patients with diabetes who have preserved renal function, anemia is more prevalent than in matched non-diabetic individuals [7, 9]. While the pathogenesis of anemia in diabetes is multifactorial, an important contributor is iron deficiency (ID) [10]. Absolute ID in individuals with diabetes can arise from poor dietary intake and/or gastrointestinal absorption as a result of gastroparesis [11, 12] or bleeding, mostly of gastrointestinal origin [13] or ulcerations of the feet [14]. Even if iron stores are adequate, functional ID may be present [15], caused by hepcidin-induced inhibition of iron export from tissue stores due to upregulation of inflammatory cytokines [16], and by hyporesponsiveness to erythropoietin [17]. However, data are sparse concerning ID and its association with anemia in patients with diabetes. The aim of this study was to determine the prevalence and characteristics of ID in a population of non-dialysis dependent patients with type 2 diabetes excluding patients with foot ulcerations and those who were treated with iron therapy or erythropoiesis stimulating agent (ESA) therapy, and to assess its association with anemia and diabetic complications.
2. Methods
2.1. Study Design
- This was a cross-sectional study of patients managed at an outpatient clinic at the Clinical Center for Diabetes, Nutrition and Metabolic Diseases in Cluj-Napoca, Romania, during 2009 to 2011. Written consent was obtained from all patients and the study was performed according to the principles of the Declaration of Helsinki concerning clinical studies.
2.2. Patient Population
- Consecutive male or female patients with type 2 diabetes were screened. Inclusion criteria were age 40–75 years and a minimum of two years since diagnosis of type 2 diabetes. Exclusion criteria were previous treatment with iron therapy or ESA, current dialysis therapy, acute metabolic disturbances, a recent history of blood loss from surgery or other non-physiological causes, amputation during the previous year, acute illness, neoplasia, endocrine disease or other chronic conditions known to potentially affect iron metabolism.
2.3. Evaluation
- Patient data and family history were obtained from medical records and during a structured interview at study entry. A venous blood sample was drawn at the following visit (up to one week later) following an overnight fast (8–10 hours). Samples were stored at -20oC and analyzed locally. Serum concentrations of iron and ferritin were measured with the automated analyzer Konelab™ PRIME 30 Clinical Chemistry Analyzer (Thermo Scientific, Waltham, MA, USA). Serum concentrations of erythropoietin, transferrin and C-reactive protein (CRP) were measured using the automatic chemiluminiscent assay IMMULITE® 2000 analyzer (Siemens Healthcare Diagnostics Inc, Tarrytown, NY, USA). Other laboratory parameters were determined using standardized methods.Renal function was assessed by estimated glomerular filtration rate (eGFR), calculated by the Modification of Diet in Renal Disease (MDRD) formula [18]. The Kidney Disease Quality Outcomes Initiative (KDOQI) classification was used to categorize stages of chronic kidney disease (CKD) [19]. Urinary albumin excretion was assigned to one of four categories: (1) <20 µg/min [no microalbuminuria] (2) 20–200 µg/min [microalbuminuria] (3) >200 µg/min [macroalbuminuria] (4) >0.5 g/dL [proteinuria]. Transferrin saturation (TSAT) was calculated as 3.982 x 0.179 x serum iron x 100) / transferrin. Anemia was defined as Hb concentration <13.5 g/dL in adult men (<12.5 g/dL in men over 70 years) and <11.5 g/dL in women, based on the European Best Practice Guidelines [20]. Absolute ID was defined as ferritin <100 ng/mL, and functional ID was defined as ferritin ≥100 ng/mL and TSAT <20%. ID anemia (IDA) was defined as anemia with either absolute or functional ID. Peripheral neuropathy was categorized as sensitive, motor or autonomic. Semmes Weinstein 10 mg monofilament was used for light touch assessment, 128 Hz Reidel Seiffert tuning fork for vibration perception threshold, pinprick with Neurotip was used for pain perception assessment, motor nerve dysfunction was assessed through ankle and knee reflexes. The presence and degree of retinopathy was assessed by using Zeiss Visucam Lite Fundus Camera and interpreted by the ophthalmologist [21].
2.4. Statistical Analysis
- Normality of distribution was assessed by the Kolmogorov-Smirnov test. Difference between means was tested with the Student T-test or the Mann Whitney test (for two groups) or using analysis of variance (ANOVA) or the Kruskal-Wallis test (for more than two groups). Frequency differences were tested with Chi square test. Odds ratio (OR) values with 95% confidence interval (CI) values were computed for associations with CKD stage, urinary protein excretion and diabetic complications using the following variables: absolute ID, functional ID, IDA, all-cause anemia. The association between a dichotomous variable and several independent qualitative and quantitative variables was tested by multivariate logistic regression analysis (forward method). From logistic regression OR values with 95% CI were reported. In the logistic regression model absolute ID, functional ID, IDA or all-cause anemia were considered dependent variables and erythropoietin level, CRP level, gender, age, duration of diabetes, CKD stage (MDRD), ischemic heart disease (yes/no), dyslipidemia (high cholesterol, high triglycerides, both, neither or unknown), abdominal circumference, body mass index, neuropathy (sensitive, motor and autonomic), retinopathy (none, background retinopathy, preproliferative retinopathy or proliferative retinopathy), urinary protein (no microalbuminuria, microalbuminuria, macroalbuminuria or proteinuria), HbA1c, and levels of total cholesterol, serum creatinine, aspartate transaminase (AST), alanine transaminase (ALT) as independent variables. Pearson correlations were used to assess the correlation between continuous variables. The association between non-parametric variables was assessed with Spearman’s coefficient of correlation. Statistical analyses were performed with SPSS 19.0, (SPSS Inc., Chicago, IL, USA). P values <0.05 were considered significant.
3. Results
3.1. Study Population
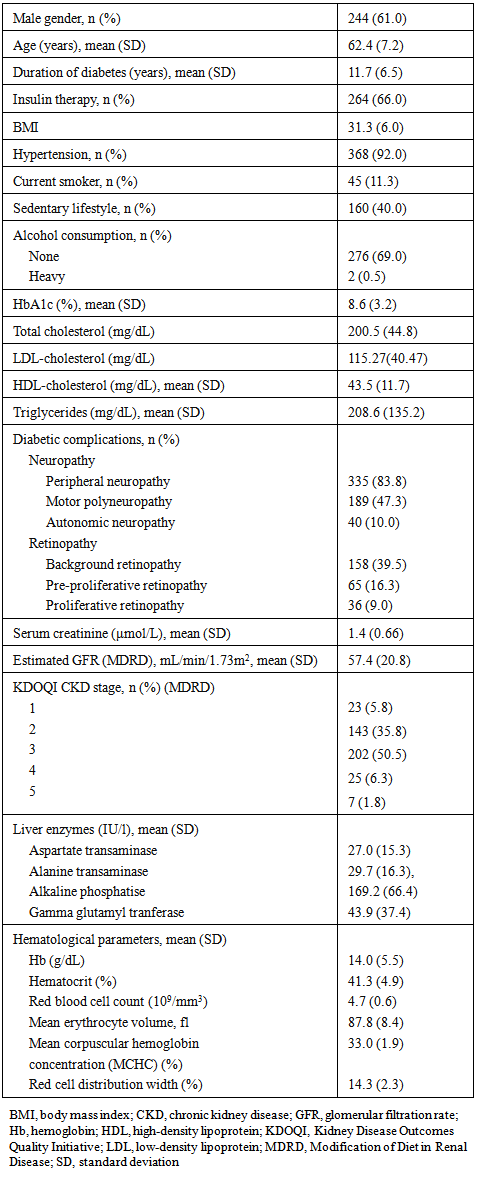
- In total, 1,443 patients were screened, of whom 400 met the eligibility criteria and were enrolled in the study. All patients were Caucasian. The majority of patients (61%) were male, with a mean (SD) age of 62.4 (7.2) years, and the mean (SD) duration of diabetes at study entry was 11.7 (6.5) years (Table 1). All except two women were post-menopausal. Two-thirds of patients (66%) were receiving insulin therapy, and mean HbA1c was 8.6% (70.6 mmol/mol). MDRD indicated the presence of CKD stages 1, 2, 3, 4 and 5 in 5.8%, 35.8%, 50.5%, 6.3% and 1.8% of patients, respectively (Table 1). CKD stages 4 and 5 were combined in a single category of severe nephropathy due to the low number of patients affected (n=32, 8.0%).
|
3.2. Iron Metabolism and Anemia
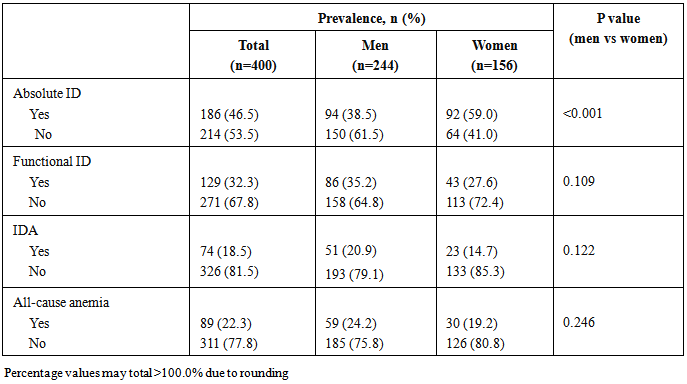
- In total, 315 patients (78.8%) were iron deficient, comprising 186 patients (46.5%) with absolute ID and 129 patients with functional ID (32.3%). IDA was present in 74 patients (18.5%), representing the majority of all anemia cases (n=89, 22.3%). Absolute ID was significantly more frequent in women than men, while the prevalence of other conditions was unaffected by gender (Table 2). Mean ferritin and TSAT were both significantly lower in patients with absolute ID (p<0.001) versus those without. Mean ferritin was significantly higher, TSAT was significantly lower, and CRP level was significantly higher in patients who had functional ID versus patients without functional ID (Table 3).
|
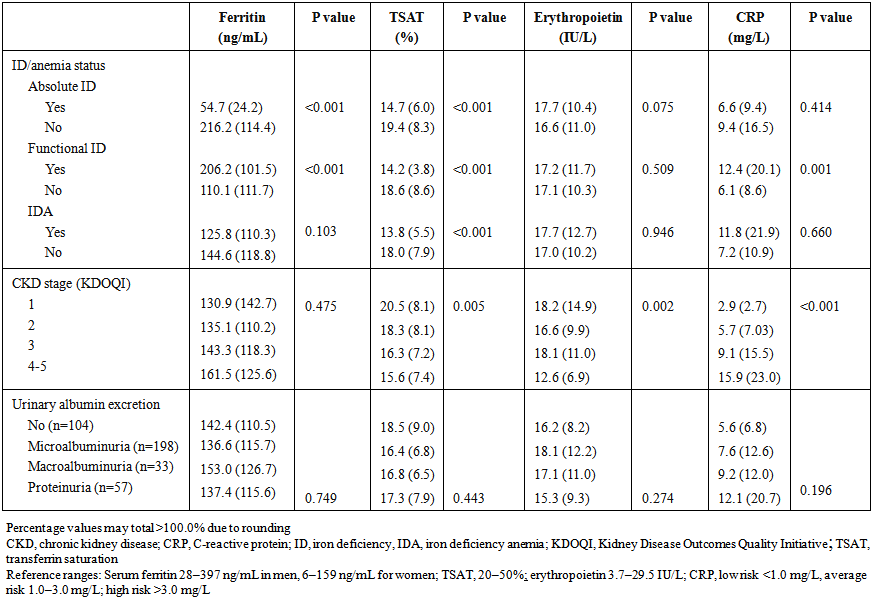 | Table 3. Serum levels of ferritin, TSAT, erythropoietin and CRP. Values are shown as mean (SD) |
3.3. CKD Severity
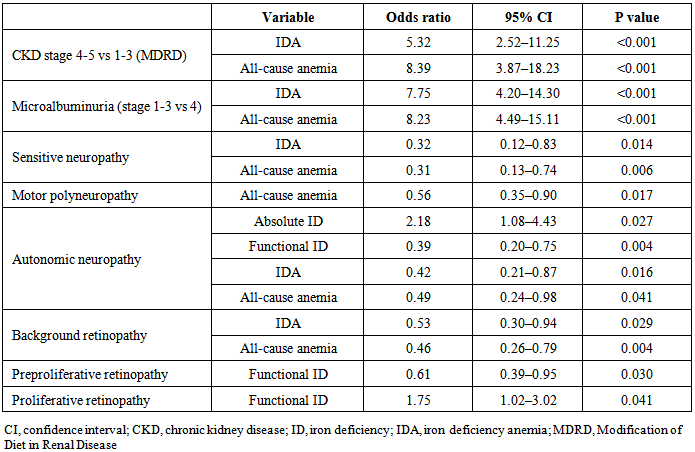
- Neither the frequency of absolute ID nor functional ID were significantly associated with CKD stage or degree of urinary protein excretion (p=0.572 and p=0.181, respectively). The prevalence of IDA and all-cause anemia, however, both increased significantly as severity of CKD increased (both p<0.001), with a markedly higher rate of both conditions in patients with CKD stage 4–5. Consistent with this, IDA and all-cause anemia also became significantly more frequent as urinary protein excretion increased (both p<0.001). There was a significant association between more severe CKD (stages 4 or 5) versus mild or moderate CKD (stages 1 or 2) and the risk of IDA (OR5.32, 95% CI2.52, 11.25; p<0.001) or all-cause anemia (OR 8.39, 95% CI3.87, 18.23; p<0.001) (Table 4).
|
3.4. Diabetes Complications
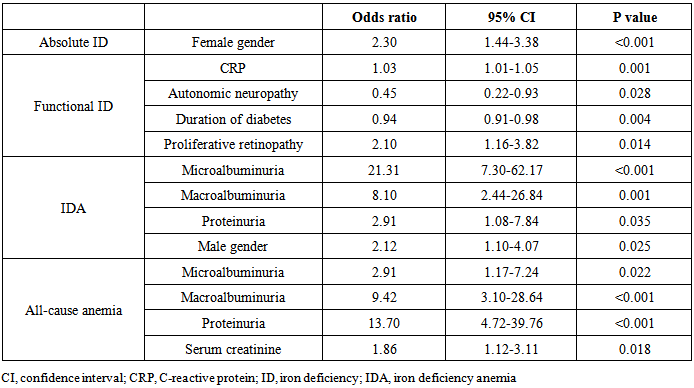
- All-cause anemia was associated with a lower risk for all types of neuropathy, IDA was associated with a lower risk for sensitive and autonomic neuropathy and functional ID was associated with a lower risk for autonomic neuropathy (Table 4). Absolute ID was associated with a higher risk for autonomic neuropathy (Table 4). Regarding retinopathy, IDA and all-cause anemia were associated with a lower risk for background retinopathy, while functional ID was also associated with a lower risk for preproliferative retinopathy, but not for proliferative retinopathy (Table 4).In logistic regression the only independent predictor of absolute ID was female gender (Table 5). Functional ID was associated with increased CRP and the presence of proliferative retinopathy. The duration of diabetes and absence of autonomic neuropathy were associated with functional ID. IDA was associated with increased urinary protein excretion and male gender. All-cause anemia was influenced by increased urinary protein excretion and increased serum creatinine (Table 5). Neither absolute nor functional ID showed a significant association with HbA1c level.
|
4. Discussion
- In this cross-sectional study of patients with type 2 diabetes who had not received iron therapy, almost 80% were iron deficient. Of these, 59% had absolute ID i.e. inadequate iron stores. IDA was present in 18.5% of patients, accounting for the majority of cases of anemia in our cohort. Overall, 22% of patients had anemia, a frequency that is within the range described in previous reports in the literature [2, 7, 9, 15, 16]. The prevalence of IDA showed a marked escalation in the prevalence of all-cause anemia in CKD stage 4–5 (Table 3), as might be anticipated given other contributory factors, notably as erythropoietin production which declines in CKD stage 4–5 [23]. The very pronounced increase in ID and all-cause anemia in severe CKD (stage 4–5) is consistent with expectations, although there was a relatively small number of patients (n=32). In the patients with severe CKD, erythropoietin deficiency was also apparent, compounding the risk of anemia in this subpopulation. A very clear direct association between urinary protein excretion and IDA or all-cause anemia was observed. This would be expected based on the progressive loss of iron within excreted proteins in the urine [24] although, surprisingly, the prevalence of absolute ID did not reflect this. Similarly, the rate of functional ID showed no clear association with renal dysfunction or urinary protein excretion. Logistic regression analysis confirmed that an increase in the level of urinary protein excretion increases the risk of IDA and all cause anemia. It seems that only severe ID manifested through anemia has an impact on diabetes status or diabetic complications. These data require confirmation in other studies to determine if this pattern is characteristic of patients with diabetes, and what mechanisms could be responsible. It should also be noted that only patients aged 40 to 75 years were included in this analysis, and results do not necessarily apply to younger individuals or, indeed, to the elderly (>75 years) in whom a variety of physiological changes, including chronic and inflammatory diseases, can influence iron storage and availability [25]. It has previously been reported that patients with diabetes who have anemia are more likely to have non-renal macrovascular complications [2]. In our analysis, worsening retinopathy and, to a lesser extent, neuropathy showed an inconsistent pattern of association with iron and Hb abnormalities (Table 4). These mixed results are unsurprising since non-renal macrovascular complications do not directly influence iron metabolism. On univariate analysis, sensitive neuropathy, motor neuropathy, autonomic neuropathy and background retinopathy were each associated with a decrease in risk of all-case anemia, and some were associated with IDA, although these relationships should not be considered causal. Only autonomic neuropathy showed a significant association with absolute ID, and proliferative retinopathy with the presence of functional ID. Patients with functional ID had a two-fold increase in CRP level, as a marker of inflammation, compared to those without, and CRP levels were higher in patients with more severe CKD (Table 3). Increased levels of proinflammatory cytokines stimulate higher levels of the acute phase protein hepcidin, which degrades the iron transport protein ferroprotein so that stored iron is not released from enterocytes and hepatocytes [26]. Consistent with this, iron stores (ferritin) were high in the patients with functional ID and in patients with CKD stage 4–5.Potential concerns about the effect of elevated iron parameters on glucose metabolism have been reported [27, 28]. In the studied population none of the patients had iron overload. However, ID also appears to be disadvantageous for outcomes in the setting of diabetes. Multivariate analysis of data from a prospective trial of 287 patients with type 2 diabetes and coronary artery disease, followed for a mean of almost four years, showed that patients with either absolute or functional ID experienced a significantly higher mortality rate than those with normal iron indices [29]. Moreover, increasing iron indices to within normal levels offers clinical benefits. In the double-blind, randomized FAIR-HF and CONFIRM-HF trials of iron deficient patients with chronic heart failure, treatment with intravenous ferric carboxymaltose was associated with an improvement in symptoms, exercise capacity and quality of life in the subgroup of patients with diabetes [30, 31]. Further controlled trials, such as the ongoing CLEVER study (NCT 01513369), are required to identify the optimal approach to iron therapy in this setting, and specifically to assess any effect of iron replenishment on HbA1c levels. Nevertheless, the high prevalence and clinical impact of iron and ID in patients with type 2 diabetes without iron therapy indicate that regular screening of iron parameters, as for other diabetes-related metabolic complications, is merited and could potentially offer clinical benefits to the patient. Funding sources: This study was supported by a research grant from Vifor Pharma. An initial draft of the manuscript was prepared by a medical writer (C Dunstall) funded by Vifor Pharma based on a Clinical Study Report provided by the authors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML