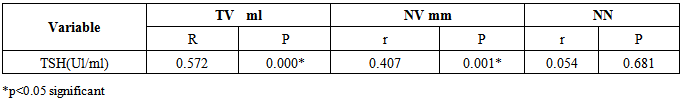
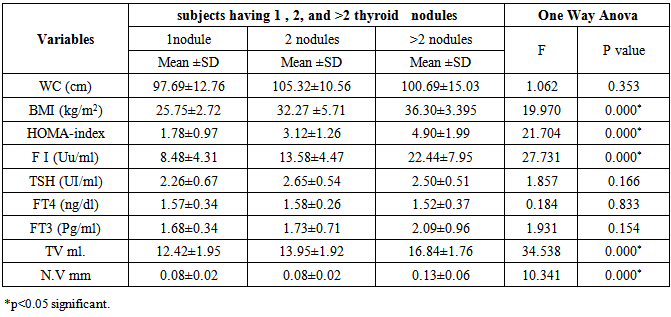
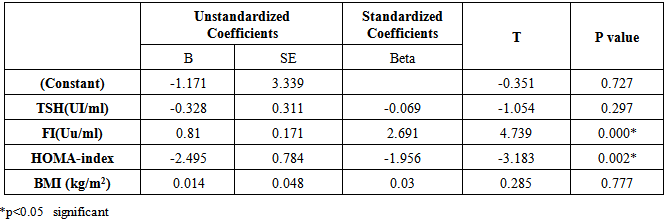
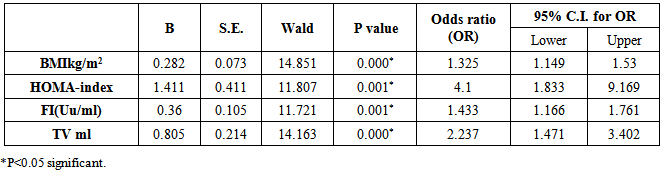
| [1] | Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care 2003; 26:3320–3325. |
| [2] | Campbell AW. The diabetes pandemic. Alternative Therapies in Health and Medicine 2011; 17 8–9. |
| [3] | Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ 2012; 344 e1369. |
| [4] | Hadaegh F, Mohebi R, Cheraghi L, Tohidi M, Moghaddam NB, Bozor- ogmanesh M, Sheikholeslami F, Azizi F. Do different metabolic syndrome definitions predict cerebrovascular events and coronary heart disease independent of their components?: 9 years follow-up of the tehran lipid and glucose study. Stroke 2012; 43: 1669–1671. |
| [5] | Vykoukal D, Davies MG. Biology of metabolic syndrome in a vascular patient. Vascular 2012; 20; 156–165. |
| [6] | Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. Journal of Clinical Investigation 2012; 122: 1316–1338. |
| [7] | Jeon CY, Haan MN, Cheng C, Clayton ER, Mayeda ER, Miller JW & Aiello AE. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care 2012; 35: 520–525. |
| [8] | Byers T, Sedjo RL. Does intentional weight loss reduce cancer risk? Diabetes, Obesity & Metabolism 2011; 13: 1063–1072. |
| [9] | Spyridopoulos TN, Dessypris N, Antoniadis AG, Gialamas S, Antonopoulos CN, Katsifoti K, Adami HO, Chrousos GP, Petridou ET. Insulin resistance and risk of renal cell cancer: a case–control study. Hormones 2012; 11 308–315. |
| [10] | Abbasi F, Brown BW, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol 2002; 40:937–943. |
| [11] | McLaughlin T, Allison G, Abbasi F, Lamendola C, Reaven G. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism 2004; 53:495–499. |
| [12] | Desser TS, Kamaya A. Ultrasound of thyroid nodules. Neuroimaging Clin N Am 2008; 18:463-478. |
| [13] | Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, et al. Society of Radiology in Ultrasound. Management of thyroid nodules detected by US: Society of Radiologist in Ultrasound Consensus Conference Statement. Radiology 2005; 237:794-800. |
| [14] | Cappelli C, Pirola I, Cumetti D, et al. Is the anteroposterior and transverse diameter ratio of non palpable thyroid nodules a sonographic criterion for recommending FNAC? Clinical Endocrinol (Oxf) 2005; 63:689-693. |
| [15] | Papini E, Guglielmi R, Bianchini A,et al. Risk of malignancy in non palpable thyroid nodules: Predictive value of ultrasound and color-Doppler features. J Clin Endocrinal Metab 2002; 87: 1941-1946. |
| [16] | WHO, Unicef, ICCiDD. Assessment of iodine deficiency disorders and monitoring their elimination, Third edition, 2007. |
| [17] | WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series 854. Geneva: World Health Organization, 1995. |
| [18] | Wang J, Thornton JC, Bari S, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutr 2003; 77:379–384. |
| [19] | Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 13:412–419. |
| [20] | Brunn J, Block U, Ruf G, Bos I, Kunze WP and Scriba PC. Volumetric analysis of thyroid lobes by real-time ultrasound. Dtsch Med Wochenschr 1981; 106: 1338-1340. |
| [21] | Usluogullari CA, Balkan F, Onal ED, Ucler R, Tam AA. Does Thyroid Volume and Nodule Formation Increase in Patients with Euthyroid Metabolic Syndrome?. Endocrinol Metab Syndr 2014; 3:3. |
| [22] | Abd El-Hafez HA, Elrakhawy MM, Abd el-Aziz S, El-Eshmawy MM. Thyroid function and volume are associated with anthropometric measurements and insulin resistance in Egyptian women with polycystic ovary syndrome. Diabetes & Metabolism 2013; 4:7. |
| [23] | Cho SW, Yi KH, HAN SK, Sun HJ, Kim YA,et al. Theraputic potential of metformin in papillary thyroid carncer in vitro and in vivo. Molecular and cellular Endocrinology 2014; 393: 24-29. |
| [24] | DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 1979; 13:E214. |
| [25] | Wallace T, Matthews D. The assessment of insulin resistance in man. Diabet Med 2002; 13:527–534. |
| [26] | Hollenbeck C, Reaven GM. Variations in insulin stimulated glucose uptake in healthy individuals with normal glucose tolerance. J Clin Endocrinol Metab 1987; 64:1169–1173. |
| [27] | DesprSs JP, Marette A. Obesity and insulin resistance. Epidemiologic, metabolic, and molecular aspects. In: Reaven GM, Laws A (eds). Insulin resistance. The metabolic syndrome X. Humana Press, Totowa, New Jersey 1999; 51–81. |
| [28] | Lakka HM, Salonen JT, Tuomilehto J, Kaplan GA, Lakka TA. Obesity and Weight Gain Are Associated with Increased Incidence of Hyperinsulinemia in Non-Diabetic Men. Horm Metab Res 2002; 34: 492–498. |
| [29] | Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000; 106: 473–481. |
| [30] | Davis FB, Thayer K, Colborn T. Significant effects of mild endogenous Hormonal changes in humans: considerations for low dose testing. Environ. Hlth Pers 2001; 109: 21-25. |
| [31] | Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T4 and T3 in normal subjects: A clue to the understanding of subclinical thyroid disease. J Clin Endocrinol. Metabol 2007; 87: 1068-1072. |
| [32] | Klieverik LP, Janssen SF, Van Riel A, Foppen E, Bisschop PH, Serlie MJ, Boelen A, Ackermans MT, Sauerwein HP, Fliers E, Kalsbeek A. Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver. Proc Natl Acad Sci 2009; 106: 5966-5971. |
| [33] | Chun H, Song K. "High normal" thyroid stimulating hormone: does it matter? Korean J. Intern. Med 2013; 28(2): 162-164. |
| [34] | Bastemir M, Akin F, Alkis E, Kaptanoglu B. Obesity is associated with increased seum TSH level .Independent of thyroid function. Swiss Medical Weekly 2007; 137:431-434. |
| [35] | Nyrnes A, Jorde R, Sundsfjord J. Serum TSH is positively associated with BMI. Int J Obesity 2006; 30:100-5. |
| [36] | Manji N, Boelaert K, Sheppard MC, Holder RL, Gough SC, Franklyn JA. Lack of association between serum TSH or free T4 and body mass index in euthyroid subjects. Clin Endocrinol 2006; 64:125-8. |
| [37] | Solanki A, Bansal S, Jindal S, Saxena V, Shukla US. Relationship of serum thyroid stimulating hormone with body mass index in healthy adults. Brief Communication 2013; 17(7): 167-169. |
| [38] | Ambrosi B, Iorio L Delnevo A Malavazos AE, Morricone L, Sburlati LF, Orsi E. Relationship of thyroid function with body mass index and insulin-resistance in euthyroid obese subjects. Journal of Endocrinological Investigation 2010; 33(Issue 9): 640-643. |
| [39] | De Pergola G, Ciampolillo A, Paolotti S, Trerotoli P, Giorgino R. Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in over- weight and obese women. Clinical Endocrinology 2007; 67: 265-269. |
| [40] | Ayturk S, Gursoy A, Kut A, Anil C, Nar A, Tutuncu NB. Metabolic syndrome and its components are associated with increased thyroid volume and nodule prevalence in a mild to moderate iodine-deficient area. Eur J Endocrinol 2009; 161: 599-605. |
| [41] | Michalaki MA, Vagenakis AG, Leonardou AS, Argentou MN, Habeos IG, Makri MG, Psyrogiannis AI, Kalfarentzos FE, Kyr-Iazopoulou VE. Thyroid function in humans with morbid obesity. Thyroid 2006; 16: 73-78. |
| [42] | Knudsen N, Laurberg P, Rasmussen LB, Bülow I, Perrild H, Ovesen L, Jørgensen T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. Journal of Clinical Endocrinology and Me- tabolism 2005; 90: 4019-4024. |
| [43] | Sari R, Balci MK, Altunbas H, Karayal-Cin U. The effect of body weight and weight loss on thyroid volume and function in obese women. Clinical Endocrinology 2003; 59: 258-262. |
| [44] | Zimmermann-Belsing T, Brabant G, Holst JJ, Feldt-Rasmussen U. Circulating leptin and thyroid dysfunction. European Journal of Endocrinology 2003; 149: 257-271. |
| [45] | Mantzoros CS, Moschos SJ. Leptin: in search of role (s) in human physiology and pathophysiology. Clinical Endocrinology 1998; 49:551-567. |
| [46] | Lewandowski K, Randeva HS, Ocallaghan CJ, Horn R, Medley GF, et al. Effects of insulin and glucocorticoids on the leptin system are mediated through free leptin. Clinical Endocrinology 2001; 54: 533-539. |
| [47] | Hegedüs L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: Current status and future perspectives. Endocr Rev 2003; 24: 102-132. |
| [48] | Rezzonico J, Rezzonico M, Pusiol E, Pitoia F, Niepomniszcze H. Introducing the thyroid gland as another victim of the insulin resistance syndrome. Thyroid 2008; 18: 461-464. |
| [49] | Rezzonico J, Rezzonico M, Pusiol E, Pitoia F, Niepomniszcze H. Metformin treatment for small benign thyroid nodules in patients with insulin resistance. Metabolic syndrome and related disorders 2011; 9: 69-75. |
| [50] | Balkan F, Onal ED, Usluogullari A, Tuzun D, Ozdemir D, et al. Is three any association between insulin resistance and thyroid cancer?: A case control case study. Endocrine 2014; 45:55-60. |
| [51] | Rendina D, De Filippo G, Mossetti G, Zampa G, Muscariello R, et al. Relationship between metabolic syndrome and multinodular non- toxic goiter in an inpatient population from a geographic area with moderate iodine deficiency. Journal of Endocrinological Investigation 2012; 35:407-412. |
| [52] | Yasar H, Ertugrul O, Ertugrul B, Ertugrul D, Sahin M. Insulin resistance in nodular thyroid disease. Endocrine Reaserch 2011; 36:167-174. |
| [53] | Zakaria E, Ghanem NS, Abd Al-Salam RF, Elshaehaby AR. The Thyroid Gland is Another Victim of the Insulin Resistance Syndrome. Med J Cairo Univ 2012; 80(1):151-158. |
| [54] | Semiz S, Senol U, Bircan O, Gumuslu S, Bilmen S, Bircan I. Correlation between age ,body size and thyroid volume in an endemic area. journal of Endocrinology Investigation 2001; 24:559-563. |
| [55] | Derwahl M, Studer H. Pathogenesis and treatment of multinodular goiter. In: Fagin J.A. (ed). Thyroid Cancer. Boston/Dordrecht/London: Kluwer 1998; 155-186. |
| [56] | Studer H, Derwahl M. Mechanisms of non- neoplastic endocrine hyperplasia a changing concept: A review focused on the thyroid gland. Endocr Rev 1995; 16: 411-426. |
| [57] | Pothiwala P, Jain SK, Yaturu S. Metabolic syndrome and cancer. Metabolic Syndrome and Related Disorders 2009; 7: 279-288. |
| [58] | Yoon DY, Chang SK, Choi CS, et al. The prevalence and significance of incidental thyroid nodules identified on computed tomography. J Comput Assist Tomogr 2008; 32(5):810-815. |
| [59] | Belfiore A, Russo D, Vigneri R, Filetti S. Graves’ disease, thyroid nodules and thyroid cancer. Clin Endocrinol (Oxf) 2001; 55(6):711-718. |
| [60] | Richardson DB. Exposure to ionizing radiation in adulthood and thyroid cancer incidence. Epidemiology 2009; 20: 181-187. |
| [61] | Gursoy A. Rising thyroid cancer incidence in the world might be related to insulin resistance. Med. Hypotheses 2010; 74: 35-36. |
| [62] | Malaguarnera F, Frasca A, Garozzo F, Giani G, Pandini V, et al. Insulin receptors isoforms and insulin –like growth factor receptor in human follicular cell precursors from papillary thyroid cancer and normal thyroid. J Clin Endocrinol Metab 2011; 96: 766-774. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML