-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2015; 4(2): 31-37
doi:10.5923/j.diabetes.20150402.02
Effect of Chemerin on Tissue Glucose Handling in Normal and Streptozotocin Induced Diabetic Rat
Moustafa H. Abdel Salam
Physiology Department, Faculty of Medicine, Zagazig University
Correspondence to: Moustafa H. Abdel Salam, Physiology Department, Faculty of Medicine, Zagazig University.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Background: Chemerin was first described as an endogenous chemokine that, through the engagement of specific receptor, attracts pro-inflammatory cells. However, it is considered now as an adipokine that can regulate adipocyte differentiation, affect adipogenesis, insulin sensitivity and was suggested to modulate cellular glucose handling; however, many of its effects remain unclear with controversial research results. The present study was performed to elucidate chemerin actions on basal and insulin-stimulated glucose handling in isolated epididymal fat, diaphragmatic strips and liver slices in normal and Streptozotocin diabetic rats. Methods: In this study, 48 adult healthy Wistar albino male rats weighing 180-240g were used to test glucose uptake in isolated epididymal fat, diaphragmatic strips and liver slices. The animals were divided into two equal main groups: Group (A) Non-diabetic and Group (B) Diabetic, Streptozotocin (STZ) induced diabetes. Each main group was further subdivided into four sub-groups Group (I): control (no drug), Group (II): treated with insulin 100 µU/ml., Group (III): treated with chemerin 100 n/ml, and Group (IV): treated with chemerin 100 n/ml and insulin 100µU/ml. Results: Chemerin administration in isolated rat epididymal fat did not cause any significant change in basal glucose uptake (mg/g wet weight/hour), while it significantly reduced insulin stimulated glucose uptake from 6.971±0.218 in to 5.964±0.3323 (p < 0.001) and from 6.098±0.373 to 5.061±0.3990 (p< 0.001) in diabetic and non-diabetic groups respectively. Similarly, in diaphragmatic strips chemerin administration had no effect on basal glucose uptake, while, it induced a significant decrease in insulin stimulated glucose uptake from 7.7421 ± 0.6531 to 5.124 ± 0.8316 (P<0.001) and from 6.993 ± 0.8316 to 3.876 ±0 .8559 (P<0.001) in diabetic and non-diabetic groups respectively. On the other hand, Chemerin administration in liver slices had no effect on glucose output (mg/g wet weight / hour), either under basal conditions or in the presence of insulin hormone. Conclusions: Chemerin administration affected insulin stimulated and not basal glucose uptake in isolated epididymal fat and diaphragmatic strips. Moreover, it has no effect on glucose handling by liver slices.
Keywords: Chemerin, Insulin, Diabetes, Glucose uptake, Diaphragm, Epididymal fat, Liver
Cite this paper: Moustafa H. Abdel Salam, Effect of Chemerin on Tissue Glucose Handling in Normal and Streptozotocin Induced Diabetic Rat, International Journal of Diabetes Research, Vol. 4 No. 2, 2015, pp. 31-37. doi: 10.5923/j.diabetes.20150402.02.
Article Outline
1. Introduction
- Chemerin is a chemo-attractant substance that was discovered in the skin and body fluids in inflammatory processes [1]. It is derived from a precursor expressed by a gene called Tazarotene-induced gene 2 (TIG2), it is secreted as an 18-kDa inactive pro-protein that can be rapidly converted by C-terminal proteolytic cleavage into its active 16-kDa form [2]. Chemerin mediates its actions via activation of a chemokine like receptor known as CMKLR1 or ChemR23, which is also highly expressed in adipose tissue [3]. Also, chemerin and its receptor; chemokine-like receptor1 (CMKLR1, or ChemR23); are highly expressed in adipose tissue [4]. Moreover, Chemerin is considered as an adipokine as it was reported to regulate adipocyte differentiation and lipolysis [5]. The serum chemerin level was associated with increased body mass index, circulating triglycerides, and blood pressure [4].The adipose tissue is widely accepted as a major endocrine active tissue that not only store energy but also is capable of producing a variety of substances that allow it to communicate with liver, skeletal muscles and regulate energy metabolism and insulin sensitivity [6].Many authors addressed the effect of chemerin on insulin sensitivity and glucose uptake, but they revealed contradicting results. Ernst et al. [7] reported that acute administration of recombinant human chemerin led to exacerbation of glucose intolerance, decreased insulin level and decreased glucose uptake by tissues. These effects were found in genetically obese (ob/ob), genetically diabetic (db/db), and diet-induced obese (DIO) but not in normo-glycemic wild type mice. Moreover, chemerin administration significantly decreased insulin stimulated glucose uptake in adipocytes [8] and in skeletal muscles as well [9].In contrast, Takahashi et al. [10] reported that chemerin led to a significant increase in insulin stimulated tyrosine phosphorylation of insulin receptors substrate and glucose uptake. Moreover, in the obese/diabetic mice, but not lean healthy controls, chemerin decreased hepatic glucose uptake, but not that of adipose or skeletal muscle [11].In vivo study revealed that chemerin deficiency enhanced muscle glucose uptake but impaired it in adipose tissue [10].Considering these results, it was obvious that chemerin can play a role in regulation of insulin sensitivity and glucose uptake in different body tissues.This study was designed to illustrate the effect of chemerin administration on glucose uptake in isolated white adipose tissue (epididymal fat), skeletal muscle tissues (isolated diaphragm) and isolated liver slices in normal and diabetic rats.
2. Material and Methods
2.1. Animals Studies
- Total number of 48 adult healthy Wistar albino male rats weighing 180-250gm was used in this study. The animals were kept in cages with a 12 hour day/night cycles and on a diet consisted of standard rat chow with free access to water throughout the study. Experiments were performed according to the guidelines of the animal ethics committee of Faculty of Medicine, Zagazig University.The animals were randomly divided into two equal major groups: Group-A (non-diabetic) and Group-B (Diabetic). Then, each major groups was also randomly subdivided into four equal subgroups (I, II, III and IV). In these subgroup epididymal fat and diaphragmatic strips were isolated and incubated for 60 minutes with Vehicle alone, insulin (100 µU/ml) [12], Chemerin (100n/ml) [10] or both, insulin (100 µU/ml) and chemerin (10n/ml) respectively. In all subgroups, glucose uptake for the isolated strips was determined.
2.2. Chemicals
- Streptozotocin: Sigma Chemical CO. ST. Louis USA. Chemerin: Sigma Chemical CO. ST. Louis USA. It was dissolved in phosphate buffer solution (PBS) Insulin: HBIOR 100 IU/ ml, SEDICO Pharmaceuticals Company®, Egypt.
2.3. Induction and Verification of Diabetes
- Animals were rendered diabetic by administration of Streptozotocin dissolved in sterile 0.1 M sodium citrate buffer (pH 4.5) just prior to use and injected intraperitoneally into rats at a concentration of 40 mg/kg body weight for 7 consecutive days. Control animals were injected with an equivalent volume of citrate buffer alone. [13]. Diabetes was verified 5 days after treatment by measuring blood glucose levels following overnight fasting using Glucometer strips and Accu-Chek Active® system (Roche Diagnostic, Germany). Animals with blood glucose levels ≥ 200 mg/dl were considered diabetic [14].
2.4. Isolation of Tissues
- The animals were anaesthetized with sodium thiopental (50 mg/kg body weight intra peritoneal). The abdominal cavity was widely opened and the liver, diaphragm and epididymal fat were removed using sharp scissors cleaned from remaining blood.Liver was also removed and slices (0.05 mm thick) were prepared using a microtome according to the method described by McKee et al [15].Tissues were placed in previously weighed flasks containing 3ml of buffer solution gassed with carbogen for 5 minutes. The flasks were closed tightly and placed for one hour in the metabolic shaker at 37°C with a rate of 120 RPM and beside these flasks two control flasks containing 3 ml of buffer only were incubated.After the incubation, period (1 hour) the flasks were cooled under tap water and samples from each flask were taken for determination of glucose content [16].Krebs-Ringer bicarbonate buffer solution (pH 7.4) was used. It consisted of NaCl 0.9 g/100ml, KCl 1.15 g/100ml, CaCl 1.22 g/100ml, KH2PO4 2.11 g/100ml, MgSO4 3.82 g/100ml, .3 g/100ml and Glucose.150 mg/100ml. In case of liver slices the solution contained glucose 100 mg/100ml. Bovine albumin was added to act as a carrier for released non-esterified fatty acids [16].
2.5. Calculation of Glucose uptake
- In order to calculate glucose uptake the following formula was used.
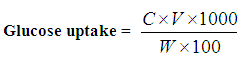 Where,C = Difference in glucose concentration between control and tissue flasks.W = Wet weight of tissue in mg.V = Volume in ml of incubation medium in the flasks.Glucose concentration was measured by enzymatic method using Coobas Integra 400 plus (Roche Diagnostic, Germany).
Where,C = Difference in glucose concentration between control and tissue flasks.W = Wet weight of tissue in mg.V = Volume in ml of incubation medium in the flasks.Glucose concentration was measured by enzymatic method using Coobas Integra 400 plus (Roche Diagnostic, Germany).2.6. Statistical Analysis
- All data were expressed as mean ± standard deviation (SD). Shapiro-Wilk and Levene median tests were performed to assess normality and equal variance, respectively. Analysis of variance (ANOVA) Test was performed to detect statistical differences between all study groups. Statistical analysis was performed using SPSS software (IBM Inc.) for windows version 20. Differences were considered statistically significant if P values were < 0.05.
3. Results
3.1. Epididymal Fat Studies
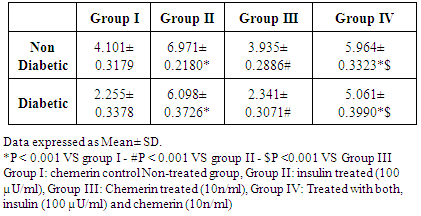
- The effect of chemerin on glucose handling by isolated rat epididymal fat in non-diabetic and diabetic animals, data expressed as mean ± SD. (Table 1 and figure 1)
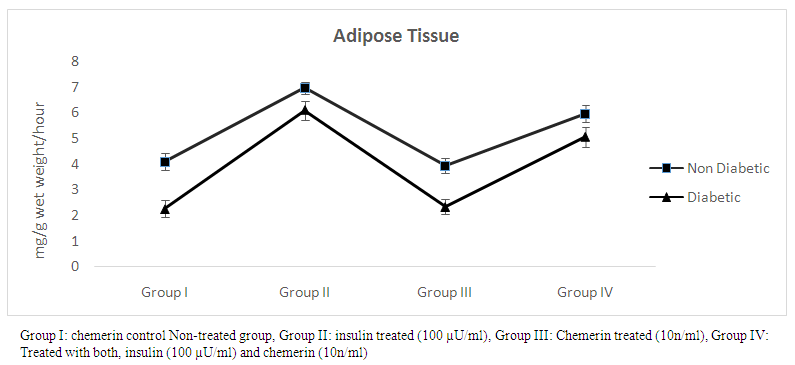 | Figure (1). Glucose uptake (mg/g wet weight/hour) in epididymal fat in control, insulin, chemerin and both insulin and chemerin treated in non-diabetic and diabetic rats |
|
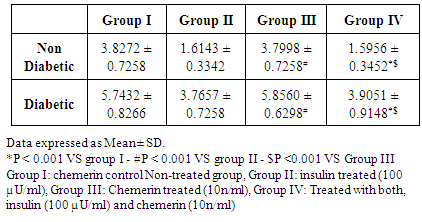
3.2. Diaphragmatic Strips Studies
- The effect of chemerin on glucose handling by isolated rat diaphragmatic strips in non-diabetic and diabetic animals, data expressed as mean ± SD. (Table 2 and figure 2)
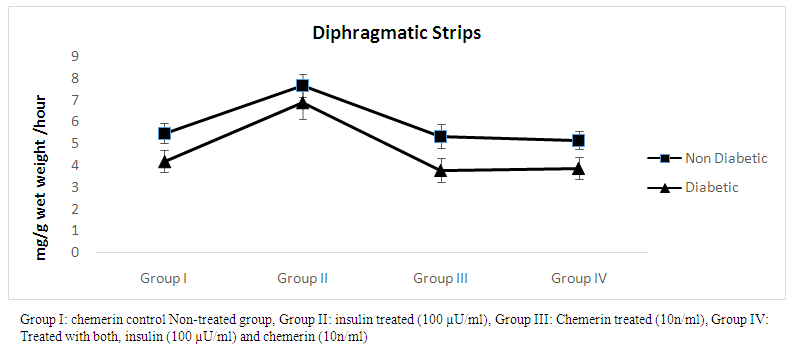 | Figure (2). Glucose uptake (mg/g wet weight /hour) in diaphragmatic strips in control, insulin, chemerin and both insulin and chemerin treated in non-diabetic and diabetic rats |
|
3.3. Liver Slices Studies
- The effect of Chemerin on glucose handling by isolated rat liver slices. (Table 3 and figure 3)
|
 | Figure (3). Glucose output (mg/g wet weight /hour) from liver slices in control, insulin, chemerin and both insulin and chemerin treated in non-diabetic and diabetic rats |
4. Discussion
- The novel adipokine chemerin is attracting good attention towards its role in regulation of glucose homeostasis and metabolism. Some authors reported elevated levels of chemerin in cases of insulin resistance as obesity and metabolic syndrome and showed positive correlations between serum chemerin level and BMI, blood pressure and serum triglycerides [17, 18]. However, they failed to show whether this increase in chemerin level was a causative factor leading to or a compensatory mechanism that try to restore these defects back to normal. In this study, it was found that chemerin administration did not affect basal glucose uptake in isolated rat epididymal fat in non-diabetic and diabetic animals, but it significantly reduced insulin stimulated glucose uptake indicating an increased insulin resistance in this tissue. These results are in agreement with Karlisch et al. [9] who reported that chemerin significantly decreased insulin stimulated glucose transport in differentiated 3T3-L1 adipocytes in vitro. Moreover, chemerin treatment was also reported to cause significant inhibition of glucose uptake in liver and white adipose tissue an effect that could be mediated via modulation of insulin signaling cascades or interference with glucose transporters functions [7].In contrast, another study reported that chemerin administration in 3T3L1 adipocytes potentiated insulin stimulated tyrosine phosphorylation of insulin receptors (IRS-1) and in turn induced a significant increase in glucose uptake and insulin sensitivity [8].Proper maintenance of blood glucose levels relies on concerted regulation of a number of metabolically important tissues. Skeletal muscle is responsible for about 80% of insulin-stimulated glucose disposal, and is a region that, when compromised, can create numerous complications for the remaining tissues in the body. Much of investigation on skeletal muscle insulin resistance has focused on lipid-induced effects [19]. In this study, it was found that chemerin pre-incubation with isolated rat diaphragmatic (skeletal muscle) strips induced a significant decrease in insulin stimulated glucose uptake in both non-diabetic and diabetic animals. These data are in agreement with a study that reported that chemerin was able in a dose dependent manner to induce insulin resistance in isolated skeletal muscles and it significantly inhibited insulin stimulated glucose uptake. However, its effect on basal glucose uptake was insignificant [9]. In the same context, another study reported that chemerin induced insulin resistance in skeletal muscle in mice on high fat diet but without affecting body-weight or lipid levels suggesting direct effects of chemerin on skeletal muscle [20].Insulin regulates sugar uptake through regulation of (GLUT4) [21]. This is mediated via production of the phosphatidylinositol 3, 4, 5 triphosphate (PIP3). [22, 23] In turn, (PIP3) downstream effects include activation of the protein kinase Akt (also known as protein kinase B) pathway. [24, 25]The effects of chemerin on skeletal muscles can be partially explained by its ability to interfere with insulin signaling pathways. It was reported that chemerin expression markedly reduced Akt1 phosphorylation and 5′AMP-Activated Protein Kinase (AMPK-α) in skeletal muscles. Activation of AMPK has been associated with stimulation of muscle glucose uptake [26, 27] and increased insulin sensitivity [28, 29]. Upstream of Akt, chemerin increased basal serine phosphorylation of IRS-1. This IRS -1 serine site is targeted by several kinases and is known to have negative effects on insulin action [10]. Both Insulin and AMPK pathways up-regulate glucose uptake in muscle via an effect of GLUT1 or GLUT4 translocation and increase in GLUT4 transcription [30, 31].GLUT2 is expressed primarily in pancreatic ß-cells, the liver and the kidneys. It is expressed in liver on the sinusoidal membrane of hepatocytes allowing for the bi-directional transport of glucose under hormonal control [32].The liver plays a unique role in the regulation of carbohydrate metabolism because it is able to both take up and release glucose. In vitro data provide strong support for the existence of auto regulation. The perfused rat liver increases its rate of glucose output when the perfusate contains no glucose or a low glucose concentration and decreases glucose output when the perfusate glucose concentration is high [33]. Low glucose Krebs solution was used to in this study in case of liver slices to enhance glucose output from liver slices. [16] In this study, it was found that chemerin has no effect on glucose output from liver slices either in non-diabetic or diabetic rats in presence or absence of insulin. These results are in agreement with Gruben et al. [34] who reported that deficiency of chemerin receptor has no effect on insulin resistance in mice.In contrast, chemerin treatment was also reported to cause significant inhibition of glucose transport in liver an effect that could be mediated via modulation of insulin signaling cascades or interference with glucose transporters functions (GLUT2) [7]. Moreover, another study reported that chemerin caused a decrease in serum insulin levels and liver glucose uptake in obese/diabetic (db/db) mice suggesting an insulin-independent mechanism that can affect GLUT2 [35].In this study, the lack of effect of chemerin on liver glucose output could be explained by the findings that only GLUT2-expressing cells (including hepatocytes) can transport STZ efficiently which make them a possible target for its cell toxicity [36]. Moreover, it was reported that GLUT2 itself is a crucial target molecule of STZ toxicity, and this toxicity seems to precede the immune reactions against β-cells [37].
5. Conclusions
- In summary, this study shows that adipose tissue and skeletal muscles are target tissues for chemerin. This adipokine significantly inhibited Insulin stimulated glucose uptake in both tissues in normal and diabetic rats. In contrast, chemerin had no effect on hepatic glucose output.Chemerin is released from adipose tissue and its level increase with BMI, so the possible role of chemerin as a link between obesity and diabetes needs to be established by further studies. In addition, the mechanisms explaining the effects of chemerin on target tissues needs further understanding and elucidation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML