-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2014; 3(4): 49-55
doi:10.5923/j.diabetes.20140304.01
Impaired Expression of ATP-Binding Cassette Transporter Genes in Diabetic ZDF Rat Blood
Richard M. Kream1, Kirk J. Mantione1, Federico M. Casares1, George B. Stefano1, 2
1Neuroscience Research Institute, State University of New York, College at Old Westbury, Old Westbury, NY, U.S.A.
2Center for Molecular and Cognitive Neuroscience, 1st Faculty of Medicine, Charles University in Prague, Prague, Czech Republic
Correspondence to: Richard M. Kream, Neuroscience Research Institute, State University of New York, College at Old Westbury, Old Westbury, NY, U.S.A..
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
In Type II diabetics and metabolically compromised adults, the pathogenesis of atherosclerosis is driven by markedly enhanced inflammatory/oxidative stress processes that involve chemotactic invasion and accumulation of activated macrophages and leukocytes and deposition of cholesterol and triglycerides within growing arterial plaques. Relatively recent studies underscore the importance of leukocyte ATP-binding cassette transporter proteins as key participants in cellular protective mechanisms that inhibit the progression of atherosclerosis, cardiovascular and metabolic diseases. We hypothesized, therefore, that ATP-binding cassette transporter gene expression would be impaired in the blood of diabetic ZDF (Zucker Diabetic Fatty) rats, as compared to healthy ZL (Zucker Lean) controls.Paired gene expression analyses were performed on whole blood samples from eleven-week old male homozygous (Fa/Fa) leptin receptor-deficient diabetic ZDF rats and their heterozygous (Fa/fa) healthy lean control ZL rats. Gene expression values were derived from DNA microarray analyses and quantified as normalized fold changes utilizing a stable reference gene. The veracity of DNA microarray data was confirmed by RT-PCR analyses of selected genes.At 11 weeks of age, ZDF rats presented a full-fledged Type II diabetic phenotype highlighted by hyperglycemia, hyperlipidemia, and liver hypertrophy. The establishment of a pathophysiological proinflammatory/oxidative stress cellular environment was monitored by differential enhancement of 3 major proinflammatory and reduction of 3 major anti-oxidative stress genes, i.e., Nfkb2, Nos2, Il1rap and Foxo3, Txnrd1, Sod2, respectively. These data were complemented by statistically significant reductions in the expression of 5 major anti-atherosclerotic ATP-binding cassette transporter genes, i.e., Abca7, Abcc1, Abcc4, Abcd3, and Abcg1. The functional impairment of ATP-binding cassette transporter gene expression in the blood of diabetic ZDF rats is consistent with profound dysregulation of metabolic homestasis leading to comorbid atherosclerotic and cardiovascular disease progression. The causal associations of these critically important membrane proteins with normative and protective anti-atherosclerotic processes underline the feasibility of potential therapeutic targeting of ATP-binding cassette transporter gene expression in metabolic disease states.
Keywords: ATP-binding Cassette Transporter, Zucker Diabetic Fatty Rat, Type II Diabetes, Pro-inflammation, Oxidative Stress, Gene expression, DNA microarray, RT PCR
Cite this paper: Richard M. Kream, Kirk J. Mantione, Federico M. Casares, George B. Stefano, Impaired Expression of ATP-Binding Cassette Transporter Genes in Diabetic ZDF Rat Blood, International Journal of Diabetes Research, Vol. 3 No. 4, 2014, pp. 49-55. doi: 10.5923/j.diabetes.20140304.01.
Article Outline
1. Introduction
- In Type II diabetics and metabolically compromised adults, the pathogenesis of atherosclerosis/arteriosclerotic vascular disease (ASVD) is driven by markedly enhanced inflammatory processes critically linked to debilitating cellular oxidative stress [1]. Mechanistically, initiation events in the pathogenesis of atherosclerosis are promoted by accumulation of polarized proinflammatory M1 macrophages with secondary recruitment of activated proinflammatory white blood cells by oxidized low-density lipoprotein (ox-LDL) deposition within the endothelial compartment of the arterial wall [2, 3]. The severity of this concerted pathophysiological process is exacerbated by the loss of insulin sensitivity, termed insulin resistance, in Type II diabetics and may be critically focused on metabolically impaired macrophage function at growing plaque sites [4]. Insulin resistant macrophages demonstrate impaired efflux and transfer of cholesterol from the endocytosis of ox-LDL to functional high-density lipoprotein (HDL), and defective clearance, termed efferocytosis, of apoptotic pro-inflammatory leukocytes. Defective efflux and transfer of internalized cholesterol from insulin resistant macrophages to HDL represents a key mechanism in the formation of pathophysiological foam cells from macrophages within growing atherosclerotic plagues and support the veracity of the LDL cholesterol /HDL cholesterol ratio as a determining risk factor in the progression of atherosclerosis [4, 5].Relatively recent studies underscore the importance of leukocyte ATP-binding cassette transporter proteins as key participants in cellular protective mechanisms that inhibit the progression of atherosclerosis, cardiovascular and metabolic diseases [6, 7]. For example, ABCA7 is a critically important ABC subfamily A gene that normally facilitates macrophage cholesterol efflux and transfer to LDL, as well as clearance of apoptotic white blood cells [8], thereby functionally inhibiting foam cell formation and atherosclerotic plaque growth [9]. Furthermore, key members of the ATP-binding cassette subfamilies C and D participate in multidrug resistance processes [34], maintenance of intracellular RedOx equilibrium and inhibition of pro-inflammatory oxidative stress [35], respectively. Members of ATP-binding cassette subfamily ABCG are critically involved in macrophage cholesterol efflux to HDL, thereby inhibiting foam cell formation [6, 7] and impairment of ABCG subfamily gene expression will contribute to accelerated human atherosclerotic disease. We hypothesized, therefore, that ATP-binding cassette transporter gene expression would be impaired in the blood of diabetic ZDF rats, as compared to healthy ZL controls, and empirically determined fold expression changes for 5 high profile candidate ATP-binding cassette transporter genes. In the present study, dependent measure data sets were derived from paired DNA microarray gene expression analyses performed on whole blood samples from eleven-week old male homozygous (Fa/Fa) leptin receptor-deficient diabetic ZDF rats and their heterozygous (Fa/fa) healthy lean control ZL rats. Cross-validation of DNA microarray data was accomplished via real-time PCR analyses of selected genes. The genetically obese diabetic ZDF rat has been well established in the biomedical literature as a high resolution translational model for elucidation of underlying pathophysiological mechanisms critically linked to advanced therapeutic development for major human disorders including Type II diabetes [10], cardiovascular disease [11], renal disease [12], and atherosclerosis [13]. To underscore the translational validity of the ZDF diabetic rat model to present a comorbid pathophysiological proinflammatory/oxidative stress cellular environment, the present study monitored expression of 3 major proinflammatory and 3 major anti-oxidative stress genes, i.e., Nfkb2, Nos2, Il1rap and Foxo3, Txnrd1, Sod2, respectively. Furthermore, the expression of 2 major leukocyte cell surface Cd8b and Cd14 genes was monitored in ZDF, as compared to non-diabetic ZL rat blood. These data were used to derive a clinically relevant model to critically evaluate the observed statistically significant reductions in the expression of 5 major anti-atherosclerotic ATP-binding cassette transporter genes, i.e., Abca7, Abcc1, Abcc4, Abcd3, and Abcg1.
2. Methodology
2.1. Experimental Animals
- Seven-week old, male homozygous (Fa/Fa) leptin receptor-deficient ZDF rats (n=10) and their heterozygous (Fa/fa) healthy lean control ZL rats (n=10) were purchased from Charles Rivers Laboratories (Wilmington, MA) and maintained by PhysioGenix, Inc. (Waukesha, WI). Animal care and all technical procedures were performed PhysioGenix, Inc. staff in accordance with IUCAC approval and established protocols described in Guide for Care and Use of Laboratory Animals (Eighth Edition). Animals were housed at 2 per cage and maintained in the Innovive caging system (San Diego, CA) upon arrival at PhysioGenix, Inc and were allowed an acclimation period of 4 days prior to baseline blood collections. Cages were monitored daily to ensure the Innovive system maintained 80 air changes per hour and positive pressure. Rat rooms were maintained at temperatures of 66-75 degrees Fahrenheit and relative humidity between 30% and 70%. The rooms were lit by artificial light for 12 hr per day (7:00 AM - 7:00 PM). Animals had free access to water and Purina 5008 rodent food (Waldschimdt’s, Madison, WI) for the duration of the study.The study lasted for 28 days and ZDF diabetic and ZL lean healthy control animals were euthanized by isoflurane overdose and thoracotomy at 11 weeks of age. For molecular biological analyses, blood was collected via descending vena cava into tubes containing RNAase inhibitor lysis buffer, immediately frozen at -20°C, and shipped on ice packs to the SUNY Neuroscience Research Institute. For analyses of serum lipids (total cholesterol, triglycerides, fractionated HDL and LDL cholesterol), blood was collected via descending vena cava and processed serum was frozen at -20°C and shipped to Comparative Clinical Pathology Services, LLC. For each study animal, day 28 fasted blood glucose was quantified using a Bayer Contour glucometer. After sacrifice, liver and abdominal fat were collected and weighed. Ten rats from each group were used in this study.
2.2. DNA Microarrays
- Total RNA extracted from rat blood samples (n=7, from each group) was isolated using the PAX RNA kit (Qiagen, Valencia, CA). DNA microarray analyses were performed using a system provided by Agilent. Arrays included four arrays per chip (Agilent 4X44K chip). Total RNA was reverse transcribed (1000 ng) using T7 primers and labeled and transcribed using Cyanine-3 dye. Each array was hybridized with at least 1.65 μg of labeled cRNA at 65°C for 18 hours. Arrays were scanned using an Agilent array scanner. The microarray platform has been previously determined to reliably quantify a minimum 1.5 fold change in gene expression. For all ZDF and ZL data sets, raw fluorescent signals were normalized to those of an empirically validated and stably expressed reference gene [14], i.e., Ppia (rat leukocyte peptidylprolyl isomerase A, also termed cyclophilin A), and paired gene expression fold changes were quantified using GeneSpring version 12.6.1 (Agilent, Santa Clara, CA). Statistical significance of paired ZDF/ZL group data was determined by calculating corrected (Benjamini-Hochberg) p-values of 2-way ANOVA.
2.3. Real Time Polymerase Chain Reactions
- Representative Real-time PCR analyses of forkhead box O3 (Foxo3) and mitochondrial superoxide dismutase 2 (Sod2) gene expression in the blood of ZDF (n=7) and ZL control rats (n=7) were performed to validate the DNA microarray data sets. Ppia was used as a reference gene. The real-time PCR master mix included 25 μL 2x universal master mix, 2.5 μL 20x detector set (with the primer and probe), and 21.5 μL of water. PCR was performed in an Applied Biosystems 7500 sequence detection system. The thermocycler conditions included denaturation at 95 °C for 15 seconds and annealing/extension at 60 °C for 60 seconds. Forty cycles of PCR were preceded by 95 °C for 10 minutes. Reactions were performed in triplicate and all 7 rat RNA samples used to generate the microarray data sets were evaluated and averaged. The relative quantities of genes were determined using the formula 2-∆∆Ct using the Applied Biosystems 7500 software.
3. Results
3.1. Presentation of a Type II Diabetic Phenotype in ZDF Rats
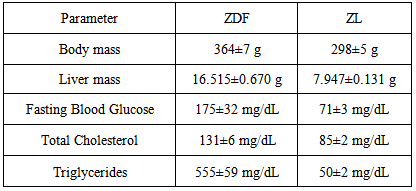
- At sacrifice, fasted blood glucose concentrations of ZDF rats were calculated to be 175±3 mg/dL, an approximate 150% increase in fasted blood glucose values as compared to non-diabetic ZL control rats, and clinically indicative of a diabetic phenotype (Table 1). In ZDF rats, the presentation of a full-fledged Type II diabetic phenotype was also supported by statistically significant increases in serum concentrations of total cholesterol and triglycerides, as compared to serum values of ZL controls. The ostensible doubling of liver mass in ZDF vs ZL control rats indicates diabetes-related abnormal intrahepatic lipid storage functionally linked to incipient steatotic liver disease [15]. In sum, basic physiological and clinical chemical parameters presented here support the validity of the ZDF rat as a high resolution translational model of Type II diabetes in humans [11].
|
3.2. Pathophysiology Linked to Inflammation
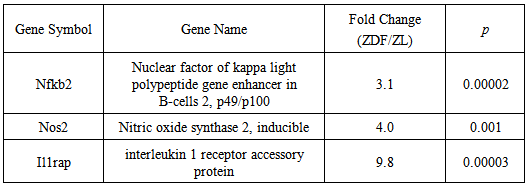
- As depicted in Table 2, DNA microarray analyses yielded a marked, statistically significant, elevation in the expression of 3 major pro-inflammatory genes in ZDF, as compared to non-diabetic ZL rat blood. The dramatic enhancements of Nfkb2, Nos2 and Il1rap gene expression, were calculated at 3.1, 4.0, and 9.8 fold, respectively, and clearly indicated a causal association of the ZDF diabetic phenotype with a pathophysiological pro-inflammatory state in blood leukocyte populations leading to accelerated atherosclerotic processes [13, 16].
|
|
3.3. ATP-binding Cassette Transporter Gene Expression in ZDF Rat Blood
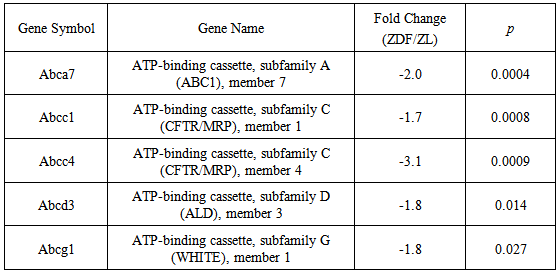
- As depicted in Table 4, DNA microarray analyses yielded a marked, statistically significant, reduction in the expression of 5 major anti-atherosclerotic ATP-binding cassette transporter genes in ZDF, as compared to non-diabetic ZL rat blood. The reductions of Abca7, Abcc1, Abcc4, Abcd3, and Abcg1 gene expression were calculated at -2.0,-1.7,-3.1,-1.8 and -1.8 fold, respectively, and clearly indicated a causal association of the ZDF diabetic phenotype with a co-ordinate impairment of protective anti-atherosclerotic processes in blood leukocyte populations [6, 7].
|
3.4. Differential enhancement in the Expression of Leukocyte Cell surface Markers
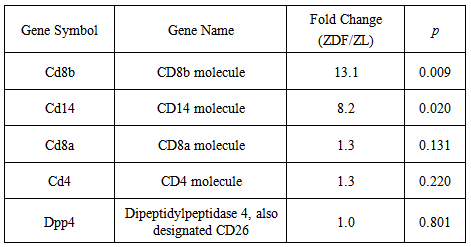
- DNA microarray analyses yielded a statistically significant, differential enhancement in the expression of 2 major leukocyte cell surface Cd8b and Cd14 genes in ZDF, as compared to non-diabetic ZL rat blood. The dramatic increases in Cd8b and Cd14 gene expression were calculated at 13.2 and 8.2 fold, respectively, and contrasted with the statistically insignificant marginal fold changes of 1.3, 1.3, and 1.0 calculated for Cd8a, Cd4, and Dpp4/CD26, respectively (Table 5). A first approximation indicates that the selective enhancements of Cd8b and Cd14 gene expression are reflective of markedly increased populations of pro-atherosclerotic cytotoxic CD8(+) T lymphocytes [19] and CD14 (+) monocytes [20, 21].
|
3.5. Validation by Real Time PCR
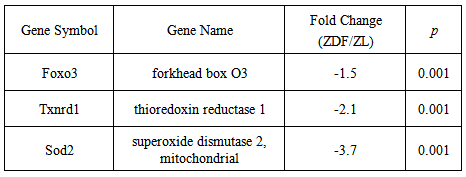
- As depicted in Figure 1, representative real-time PCR analyses confirmed the validity of the fold changes in gene expression derived from DNA microarray data sets. Real-time PCR analyses yielded reductions of 25.0±1.9% and 72.8±5.1% for Foxo3 and Sod2 gene expression, respectively, in ZDF, as compared to non-diabetic ZL rat blood. Independent 2 sample t-tests demonstrated that the genes were differentially expressed (Figure 1). The comparative reductions in Foxo3 and Sod2 gene expression were consistent with the respective fold changes of -1.5 and -3.7 derived from DNA microarray data sets (Table 3).
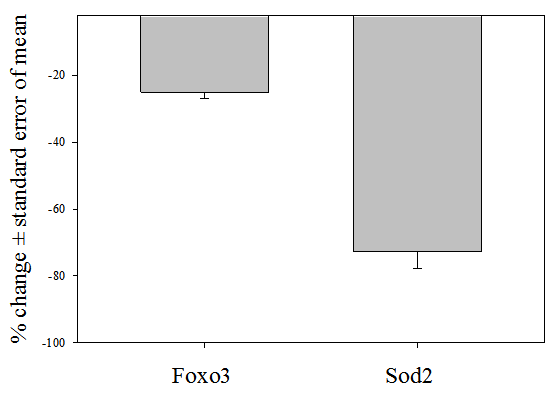 | Figure 1. Representative real-time PCR analyses of forkhead box O3 (Foxo3) and mitochondrial superoxide dismutase 2 (Sod2) gene expression in ZDF rat blood |
4. Discussion
- Atherosclerosis and CVD are major co-morbid complications of untreated Type II diabetes in diverse human populations [1, 22, 23]. In the presently employed ZDF rat model of Type II diabetes, severe dysregulation of triglyceride and cholesterol metabolism is functionally linked to a chronic pro-inflammatory state characterized by enhanced cellular oxidative stress [24], resulting in dramatically enhanced pathophysiological processes. The translational depth of the chosen ZDF rat model is amply validated by the functional association of diminished insulin sensitivity and dysregulated lipogenesis and cholesterol metabolism. A careful perusal of the biomedical literature reinforces the veracity and translational applicability of the employed in vivo animal model through multiple pathophysiological linkages to the etiology and progression of severe atherosclerosis. The markedly enhanced expression of 3 major pro-inflammatory genes established a causal association of the ZDF diabetic phenotype with a pathophysiological pro-inflammatory state in blood leukocyte populations leading to accelerated atherosclerotic processes. Nfkb2 encodes a subunit of the transcription factor complex NFkB that is expressed by numerous cell types and functions as a central activator of genes involved in inflammation and immune function [25]. Accordingly, the observable marked enhancement of Nfkb2 gene expression is consistent with clinically established Type II diabetic co-morbidity leading to pro-inflammatory-mediated atherosclerotic lesion formation [1]. The Ilrap gene encodes the interleukin 1 receptor accessory protein. The expressed protein is an essential subunit of the interleukin 1 receptor complex which initiates signaling events that result in the activation of interleukin-1 responsive pro-inflammatory genes [26]. Metabolic and co-morbid CVD disorders such as atherosclerosis and ischemic heart disease are characterized by uncoupling physiological constitutive NO production leading to marked enhancement of pro-inflammatory Nos2 gene expression [27]. Excess NO production and release has been demonstrated to promote the progression of atherosclerosis and genetic deletion of Nos2 has been shown to increase the serum capacity of reverse cholesterol efflux in the aortas of apoE(-/-) mice [28].Oxidative stress is a hallmark of Type II diabetes and related metabolic diseases and represents a significant risk factor for atherosclerosis [29]. Accordingly, the markedly reduced expression of 3 major anti-oxidative stress genes reinforced a causal association of the ZDF diabetic phenotype with a pathophysiological oxidative stress leading to accelerated atherosclerotic processes. Type II diabetic mice have been observed to have markedly elevated mitochondrial reactive oxygen species in conjunction with significantly reduced mitochondrial Sod2 protein expression in coronary endothelial cells [30]. Accordingly, the observed dramatic reduction in Sod2 gene expression may be functionally lined to deleterious cellular oxidative stress. Txn1 (Thioredoxin 1) and its cognate reductase, thioredoxin reductase 1, regulate cellular reduction/oxidation (redox) status. The impairment of normative cellular redox state via decreased Txnrd1gene expression in diabetic ZDF rat blood will negatively affect multiple cellular pathways, which significantly contribute to the pathogenesis of atherosclerosis [16, 18]. Foxo3 is a direct transcriptional regulator of a key group of oxidative stress genes including Sod2 that interact to inhibit oxidative damage within the vascular endothelium [17]. Importantly, myeloid cell proliferation and oxidative stress can be significantly inhibited via the Foxo3 branch of normative insulin receptor signaling, thereby reducing predisposition to atherosclerosis [31, 32].In light of the above, the negative functional consequences of state-dependent impairment of ATP-binding cassette gene expression are profound and compelling. The protein expressed by the ABCA7 gene normally facilitates macrophage cholesterol efflux and transfer to LDL, as well as clearance of apoptotic white blood cells [8]. Importantly, deleterious foam cell formation was enhanced after oxidized LDL exposure that was functionally linked to down regulation of ABCA7 [9] and brief exposure of monocytes to a low concentration of oxidized LDL was observed to induce a long-lasting proatherogenic macrophage phenotype characterized by increased proinflammatory cytokine production, decreased ABCA7 expression and foam cell formation [33]. In light of these data, a reduction of ABCA7 gene expression will functionally promote accelerated pro-atherosclerotic processes.Members of the ATP-binding cassette subfamily C are primarily involved in multidrug resistance processes. The protein expressed from the ABCC1 gene has been demonstrated to inhibit NOS2 expression in polarized macrophages thereby suggesting a role for ABCC1 transporters as anti-inflammatory mediators [34]. The protein expressed from the ABCC4 gene is involved in cellular detoxification processes as an organic anion pump and appears to represent a critical factor in normative platelet function [35]. The ATP-binding cassette transporter ABCD1 is an integral peroxisomal protein involved in maintenance of intracellular RedOx equilibrium and inhibition of oxidative stress [35]. Impairment of ABCD1 gene expression will promote significant pro-atherosclerotic effects via stimulation of pro-inflammatory oxidative stress. The ATP-binding cassette transporter ABCG1 is critically involved in macrophage cholesterol efflux to HDL, thereby inhibiting foam cell formation [6, 7]. Reconstituted HDL infusions have been demonstrated to act via an ABCG1-dependent mechanism to limit hypercholesterolemia-driven excessive platelet production, thrombosis, and atherogenesis. Accordingly, impairment of ABCG1gene expression will markedly compromise transporter-dependent cholesterol efflux pathways in polarized macrophages leading to accelerated human atherosclerotic disease.Finally, the observed differential enhancements in the expression of leukocyte cell surface marker Cd8b and Cd14 genes in ZDF rat blood reinforce the presentation of a co-morbid deleterious pro-inflammatory, oxidative stress, cellular environment. Cytotoxic CD8(+) T lymphocytes represent up to 50% of leukocytes in advanced human atherosclerotic plaques and are causally associated with increased lipid and macrophage accumulation, apoptotic cells and necrotic cores [19, 36]. Furthermore, elevated concentrations of cytotoxic CD8(+) T lymphocytes have been demonstrated to vary inversely with concentrations of anti-inflammatory regulatory T cells (Tregs) that play a critical role in modulating disease-related tissue inflammation [37]. Interestingly, the presently observed reduction of Foxo3 gene is consistent with a functional down-regulation of Tregs in ZDF rat blood [31, 32].Monocyte recruitment to inflamed arterial endothelium has been shown to initiate atherosclerotic plaque formation. CD14 encodes a monocyte/macrophage surface antigen associated with subsets of pro-inflammatory monocytes in the peripheral circulation [20]. In patients with hypertriglyceridemia, increased pro-atherosclerotic monocyte adhesion was attributable to CD14-enriched monocyte populations [21]. In sum, the observable enhancement of CD14 gene expression supports the functional role of activated monocytes in atherosclerotic processes.
5. Conclusions
- The functional impairment of ATP-binding cassette transporter gene expression in the blood of diabetic ZDF rats is consistent with profound dysregulation of metabolic homestasis leading to comorbid atherosclerotic and cardiovascular disease progression. Additionally, internal validation of the study emerges in the changes in gene pattern expression since it consistently supports the hypothesis that proinflammation allows for the potential of other disorders to be manifested. The causal associations of these critically important membrane proteins with homeostatic and protective cellular processes underline the feasibility of potential therapeutic targeting of ATP-binding cassette transporter gene expression in metabolic disease states.
ACKNOWLEDGEMENTS
- The authors wish to thank Joshua M. Samuel for his expert technical assistance.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML