-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2014; 3(3): 36-40
doi:10.5923/j.diabetes.20140303.02
Hypoglycemic and Nephroprotective Effect of Cinamomum Cassia on Alloxan Induced Diabetic Mice
Ranjit Kumar1, Asif Iqubal2, Arun Kumar Singh2, A. Nath1, J. K. Singh1, Md Ali1, Arun Kumar1
1Mahavir Cancer Institute & Research Centre, Phulwarisharif, Patna (Bihar), India
2B. D. College, Patna Magadh University, Bodh Gaya, Bihar
Correspondence to: Ranjit Kumar, Mahavir Cancer Institute & Research Centre, Phulwarisharif, Patna (Bihar), India.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Diabetes mellitus is a disease associated with hyperglycaemia. Diabetes mellitus is a chronic disease, it generates overproduction of free radicals & reactive oxygen species (ROS), which trigger on oxidative stress, enhanced lipid per oxidation, damage to DNA & protein degradation. Cinnamon is a traditional herbal medicine to treat various diseases. Cinnamon has polyphenolic components, which is antioxidative agents, reactive oxygen & nitrogen species scavengers, redox- active transitional chelators & enzyme modulators. The present study is designed to evaluate hypoglycaemic and nephroprotective effect of Cinamomum cassia on biochemical and histological parameters of kidney of mice. The control group of mice received distilled water orally. The ‘treatment’ groups received alloxan 125 mg/kg b.w by intra-peritoneal method once followed by four weeks administration of aqueous extract of bark of Cinamomum cassia orally through Gavage method. Glucose level increases many fold in alloxan induced diabetic mice. Urea, uric acid and lipid per oxidation level were increases many fold. Glomerulus, Bowman’s capsule, PCT and DCT were degenerated in alloxan induced diabetic group of mice. Cinnamon administered group show restoration in both biochemical and histological parameters of kidney of mice. It is concluded that Cinamomum cassia acts effectively against alloxan induced diabetes and maintains normal glucose level effectively. It also acts against nephrotoxicity and maintains biochemical parameters of kidney and LPO to normal level. Amelioration in the kidney tissue is observed, which denotes the normal functioning of kidney. It is evident that Cinamomum cassia has effective hypoglycaemic and nephroprotective effect.
Keywords: Hypoglycaemic, Nephrotoxicity, Cinamomum cassis, Alloxan
Cite this paper: Ranjit Kumar, Asif Iqubal, Arun Kumar Singh, A. Nath, J. K. Singh, Md Ali, Arun Kumar, Hypoglycemic and Nephroprotective Effect of Cinamomum Cassia on Alloxan Induced Diabetic Mice, International Journal of Diabetes Research, Vol. 3 No. 3, 2014, pp. 36-40. doi: 10.5923/j.diabetes.20140303.02.
Article Outline
1. Introduction
- Diabetes mellitus (DM) is a chronic metabolic disease associated with hyperglycaemia. This occurs due to defective Insulin secretion or function. The prolonged hyperglycaemia accompanying diabetes causes tissue damage, which results in degenerative complications in many organs including kidney, heart, muscles, eye, liver, & many other organs [1]. DM is affected to about 5.4% of the world population by the Year 2025 [2]. Mortality rate are very drastically change due to diabetes. Mortality in case of cardiovascular with diabetes is approximately doubled than non- diabetic cardiovascular & triple in case of renal failure [3]. It may causes alteration in thyroid hormones system by reduction in the TSH stimulation of thyroid gland & reduction in generation of T3 from T4 hormone. There is alteration in secretary activity of thyroid gland & pituitary gland by change in their structural configuration [4, 5]. DM also affected nervous system of our body. This condition is called diabetic neuropathy. Sensory motor & autonomic function are affected due to metabolic changes in diabetic neuropathy [6]. Diabetes mellitus is a chronic disease due to persistent & long term it generates overproduction of free radicals & reactive oxygen species (ROS), which trigger on oxidative stress, enhanced lipid per oxidation, damage to DNA & protein degradation [7].Cinnamon is a traditional herbal medicine to treat various diseases. It has antityrocinase activity. It acts like antimutagenic activities [8, 9]. Cinnamon has polyphenolic components, which is antioxidative agents, reactive oxygen & nitrogen species scavengers, redox- active transitional chelators & enzyme modulators [10].Thus, the present study is designed to evaluate hypoglycaemic and nephroprotective effect of Cinamomum cassia on biochemical and histological parameters of kidney of mice.
2. Materials and Methods
- Animals: The mice (Mus musculus) were reared in our laboratory. The age group of mice selected for the study was 8 weeks old with 30±2 gm. body weight (b.w)Chemicals: Alloxan, manufactured by Excel India Pvt. Ltd., Mumbai was utilized for the experiment. Medicinal plant: Aqueous bark extract of Cinamomum cassia is administered orally to diabetic group of mice. Fresh bark of Cinamomum cassia was purchased from local herbal store in Patna, India. It is identified by Dr Ramakant Pandey, Department of Botaney, Patna University, Patna.Study groups & sampling: The control group of 6 mice received distilled water orally. The ‘treatment’ groups (n=6) received alloxan 125 mg/kg b.w by intra-peritoneal method once followed by eight weeks administration of aqueous extract of bark of Cinamomum cassia (70 mg/kg/b.w/day) orally through Gavage method. Animals were sacrificed after the scheduled treatment. Serum was collected for urea, uric acid lipid per oxidation and glucose estimation. The kidney from all the animals were removed and washed three times in isotonic saline (0.85 v/w %) and fixed in neutral formalin for Light Microscope (LM) study.
3. Results
- Body weight of control mice was 29.33 ± 1.21 gm in alloxan induced diabetic group of mice it was 27.81±0.89 gm. It was 28.5 gm in diabetic control group while 34.27 ± 2.34 gm in cinnamon four weeks administered group.Fasting glucose level in control group of mice was 89.33 ± 3.38 mg/dl. In diabetic group of mice fasting glucose level was 209.3 ± 8.64 mg/dl. Glucose level was 139.7 ± 1.85 mg/dl and 105.0 ± 1.73 mg/dl in Cinamomum cassia two weeks and four weeks administered group of mice (Graph: 1).
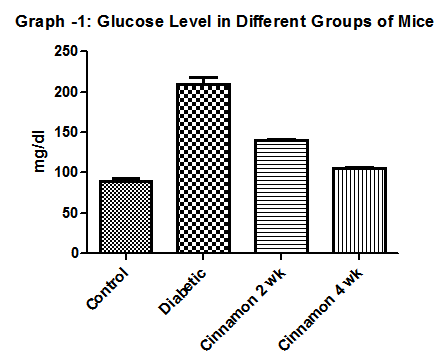 | Graph 1. Show Glucose level in different group of mice. N=6 in each group and p < 0 .05. In diabetic control group it was 201.4±3.61 mg/dl |
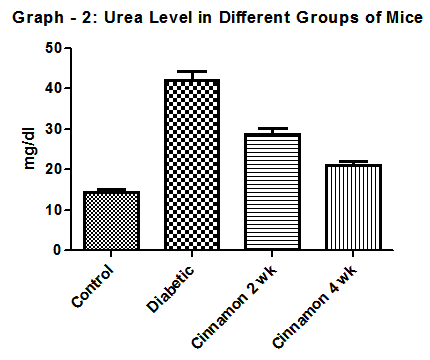 | Graph 2. Show Urea level in different group of mice. N=6 in each group and p < 0 .05. In diabetic control group it was 39.5± 1.24 mg/dl |
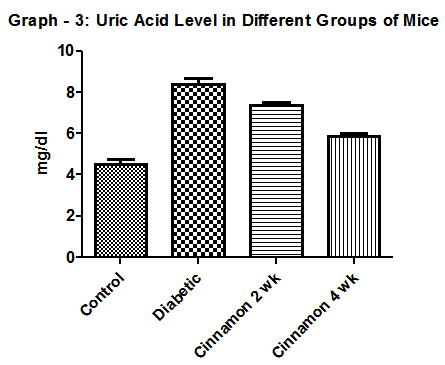 | Graph 3. Show Uric acid level in different group of mice. N=6 in each group and p < 0 .05. In diabetic control group it was 8.1 ± 0.48 mg/dl |
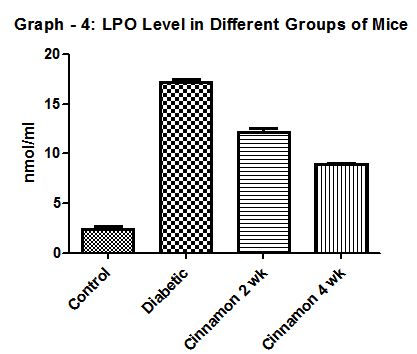 | Graph 4. Show Lipid peroxidation level in different group of mice. N=6 in each group and p < 0 .05. In diabetic control group it was 15.8 ± 1.08 nmol/ml |
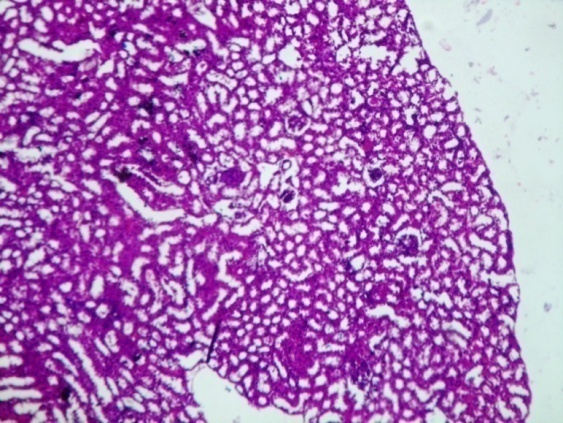
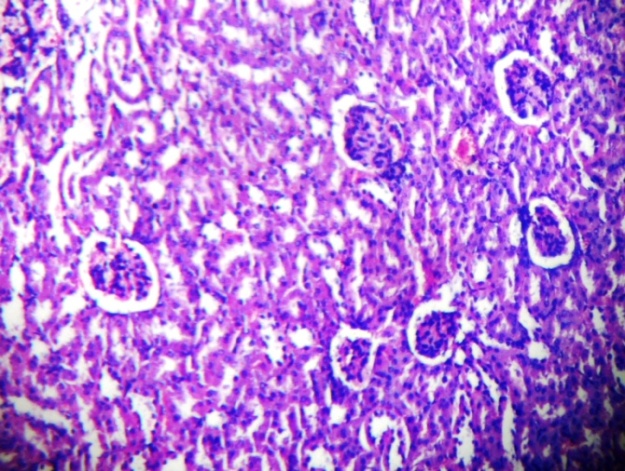 | Figure 1. Show kidney of control mice with well defined glomerulus and bowman’s capsule. Proximal convoluted tubule (PCT) and Distal Convoluted Tubule (DCT) was normal |
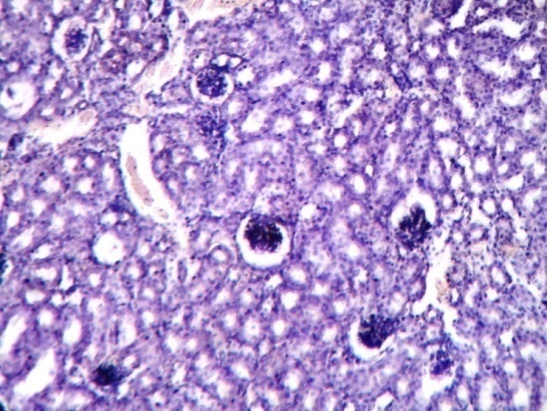
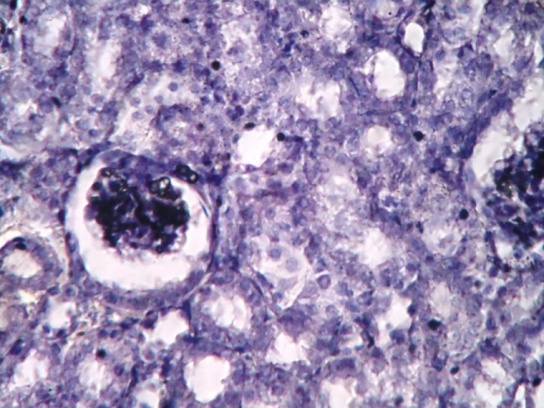
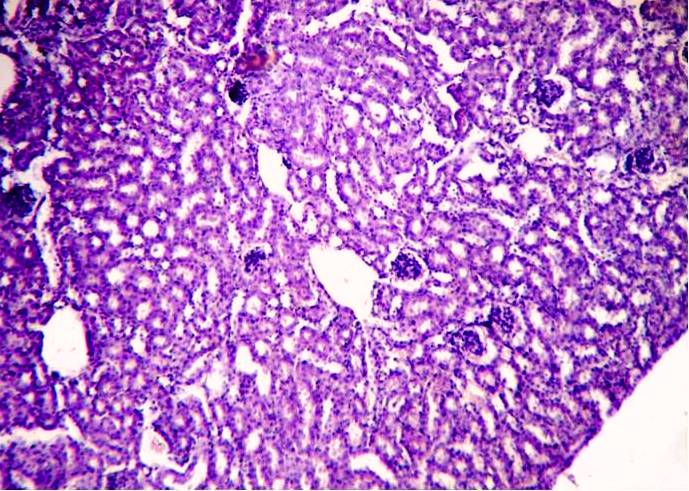 | Figure 2. Show kidney of diabetic mice with many vacuolated spaces in cortex region of kidney. Dilated bowman’s capsule was seen |
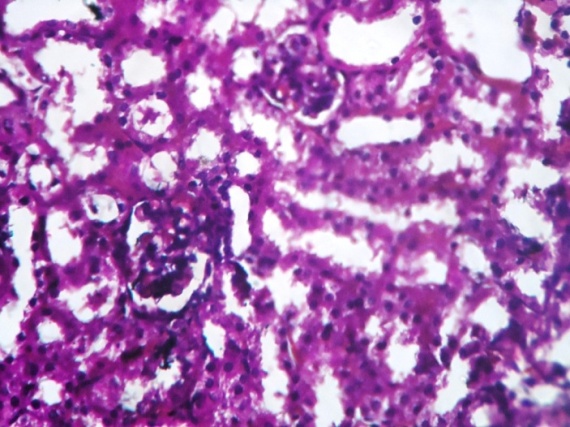
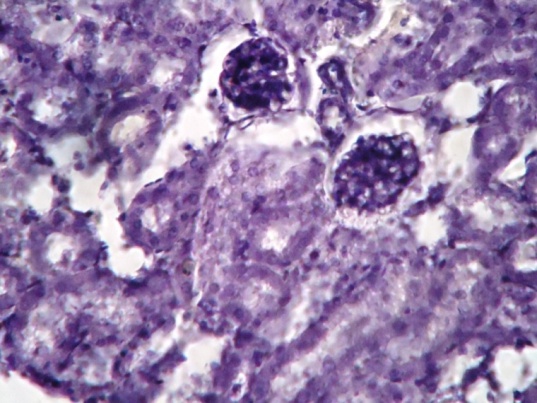 | Figure 3. Show kidney of diabetic mice with degenerated nuclei of PCT and DCT. Rudimentary cytoplasm of PCT and DCT was also observed. Degenerated Bowman’s capsule was observed |
4. Discussion
- Diabetic hyperglycemias induce certain biochemical parameter such as increased blood urea production in diabetes by enhancing catabolism of liver & plasma protein. This elevation of plasma level of urea & creatinine are marker of renal dysfunction [11].In diabetic condition the morphology of podocyte is abnormal. Podocyte is the positively charged basement membrane on the glomerular surface. Therefore proteinuria, glomerulosclerosis occur as secondary response [12].In diabetes mellitus increase the production of free radicals which cause damage to cellular proteins membrane lipids & nucleic acid & in long term it trigger cell death pathway [13, 14]. In present study lipid per oxidation was also observed increased. Glucose level was increased many fold in present study.In diabetes blood urea level increases & reason behind this is due to enhanced catabolism of both liver & plasma proteins. Cinnamon has hepatoprotective effect against liver injury & oxidative stress [15]. Urea and uric acid was increased many fold. Kidney tissues were degenerated to greater extent with rudimentary glomerulus and Bowman’s capsule. PCT and DCT were also observed in degenerated condition in diabetic group of mice.In rats cinnamon enhanced glucose uptake by stimulation insulin receptor beta, insulin receptor substrate-1 & phosphatidylinositol-3 kinase [16]. In cinnamon administered mice group glucose level was decreased effectively. LPO, Urea and uric acid level was also restored to greater extent in cinnamon administered group.Cinnamon extract contains biological active substances such as cinnamic aldehyde, cinnamic acid, tannin & methyl- hydroxychalcone polymer. Cinnamon extract decreases blood glucose & lipid profiles in rats & increases insulin sensitivity & glucose uptake in adiposities. Cinnamon extract has insulin mimetic properties. Dried aqueous extracts of Cinnamon has potentiate insulin regulated glucose utilisation by enhancing insulin signalling [17]. In present study kidney were restored to greater extent in cinnamon administered group of mice. Nucleus and cytoplasm material of both glomerulus and Bowman’s capsule were restored effectively.
5. Conclusions
- It is concluded from study that Cinamomum cassia acts effectively against diabetes and maintains normal glucose level effectively. It also acts against nephrotoxicity and maintains biochemical parameters of kidney and LPO to normal level. It also restores tissues of kidney. It is evident that Cinamomum cassia possesses hypoglycaemic and nephroprotective effect.
ACKNOWLEDGMENTS
- The authors are thankful to Mahavir Cancer Sansthan for providing infrastructural facility during this work. We are thankful to our entire research team who provide us every support during this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML