-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2014; 3(3): 27-35
doi:10.5923/j.diabetes.20140303.01
Antihyperglycemic and Antioxydant Properties of Alstonia boonei De Wild. (Apocynaceae) Stem Bark Aqueous Extract in Dexamethasone-Induced Hyperglycemic Rats
Barnabé Lucien Nkono Ya Nkono1, Selestin Dongmo Sokeng2, Dzeufiet Djomeni Paul Désiré3, Pierre Kamtchouing3
1Department of Biology, Higher Teacher Training College, University of Yaounde 1, Cameroon
2Department of Biological Sciences, Faculty of Science, University of Ngaoundere, Cameroon
3Department of Animal Physiology and Biology, Faculty of Science, University of Yaounde 1, Cameroon
Correspondence to: Barnabé Lucien Nkono Ya Nkono, Department of Biology, Higher Teacher Training College, University of Yaounde 1, Cameroon.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Decoction prepared from stem bark of Alstonia boonei (A. boonei) is used to alleviate the symptoms of diabetes mellitus in folk medicine. In the present study, the antioxidant and antihyperglycemic potential of the aqueous extract of A. boonei was evaluated in normal and dexamethasone phosphate-induced-hyperglycemic rats. Aqueous extract of A. boonei was administrated to wistar rats at 200 and 500 mg/kg for 30 days. Hyperglycemia was induced by repeated daily injection of dexmethasone for 30 days at the dose of 0.4 mg/kg. Administration of dexamethasone led to a significant increases in the average blood glucose level, triglycerides, total cholesterol, ALT and AST, bilirubin, creatine and urea in rats. Dexamethasone elevated the level of MDA but decreased the level of GSH and CAT. However, these increases were significantly (p<0.001) attenuated in rats orally pretreated with A. boonei water extract for blood glucose level, triglycerides and AST. A. boonei at the dose of 200 mg/kg significantly suppressed the rise of blood glucose by 36% 30 min after glucose load as compared to the dexamethasone rats. In addition, A. boonei significantly (p<0.001) improved OGTT in the treated rats. Our results infer that the aqueous extract of A. boonei steam bark demonstrated remarkable antioxidant and antihyperglycemic activities in dexamethasone-induced hyperglycemic rats.
Keywords: Alstonia boonei, Dexamethasone, Antihyperglycemia, Antioxidant, Rats
Cite this paper: Barnabé Lucien Nkono Ya Nkono, Selestin Dongmo Sokeng, Dzeufiet Djomeni Paul Désiré, Pierre Kamtchouing, Antihyperglycemic and Antioxydant Properties of Alstonia boonei De Wild. (Apocynaceae) Stem Bark Aqueous Extract in Dexamethasone-Induced Hyperglycemic Rats, International Journal of Diabetes Research, Vol. 3 No. 3, 2014, pp. 27-35. doi: 10.5923/j.diabetes.20140303.01.
Article Outline
1. Introduction
- Diabetes mellitus is a chronic disease caused by inherited or acquired deficiency in production of insulin by the pancreas or by the ineffectiveness of the produced insulin. Such a deficiency results in increased concentrations of glucose in the blood, which in turn damage many of the body’s systems, in particular the blood vessels and nerves [1]. The number of adults with diabetes in the world will rise to 300 million by the year 2025 and the major part of this numerical increase will occur in developing countries [2]. Treatment of type 2 diabetes mellitus patients with sulfonylureas and biguanides is always associated with side effect [3]. So, many herbal medicines have been recommended for the treatment of diabetes [4]. Traditionally plant based medicines are used throughout the world for a range of diabetic presentations [5]. The UK perspective diabetic study (UKPDS) had indicated that the intensive glycemic control reduced the disease complications and mortality [6]. Many Cameroonian plants have been reported by various authors to treat diabetes traditionally [7].Alstonia boonei De Wild. is a large deciduous evergreen tree, usually up to 45 m tall and 1.2 m in diameter, belonging to the family Apocynaceae. It is a native of tropical and subtropical Africa, Southeast Asia, Central America and Australia [8]. A. boonei stem bark, has been reported to possess anti-inflammatory, analgesic and antipyretic activities, antifungal, antibacterial, antiviral, antithrombosis, anti-tumor and antioxidant activities [9, 10]. Raji and Akinsomisoye [11] report that the methanolic extract of A. boonei has reversible antifertility effects in male rats. The stem bark is commonly used in treating malaria, toothache and rheumatism [8, 12]. The chloroform and methanol extracts from the roots showed activity against Staphylococcuc aureus, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa [13]. The antidiabetic and antihyperlipidemic effect of Alstonia scholaris, a specie in the same genera has also been reported [14]. The hypoglyceamic activity of stem bark aqueous extract of A. boonei was reported by Akinloye et al. [15]. However, to the best of our knowledge, there is dearth of information on the effect of this plant on blood glucose level. Dexamethasone- induced hyperglycemic rat is an animal model of type 2 diabetes mellitus [16]. The objective of this study was to evaluate antidiabetic and antioxidant effects of A. boonei stem bark in dexamethasone-induced hyperglycemic rats.
2. Material and Methods
2.1. Drugs and Reagents
- Dexamethasone sodium phosphate (Rotexmedica, Bunsenstrasse 4-22946 Trittau/Germany), glibenclamide (Sanofi Winthrop Industrie – 56, Route de Choisy-Au-Bac – 60205 Compiègne – France) and metformin (Denk Pharma GmbH & Co. KG Prinzregentenstr. 79, 81675 München, Germany) used in the experiment were all of analytical grade. Biochemical analyses were performed with commercial kit (Fortress diagnostics, UK) and blood glucose levels were measured using single touch glucometer (Accu-check, Roche Dianostics, Germany).
2.2. Plant Material and Extraction
- Fresh stem barks of A. boonei were collected from Ombessa (Cameroon) during November 2012. The botanical identification of the plant material was performed by taxonomist in the National Herbarium of Cameroon and a voucher specimen (N°43368HNC) was deposited. The stem bark were washed thoroughly with water and, dried at room temperature. The dried stem bark was milled. One hundred gram of the powder was weighed and extracted in 1000 mL of distilled water by decoction for 30 min. The aqueous extract was lyophilized and thereafter preserved at room temperature for further use.
2.3. Laboratory Animals
- Male wistar rats (Rattus norvegicus) weighing about 125-160 g were used. They were obtained from the breeding house of the Animal Physiology Laboratory, Faculty of Sciences University of Yaounde I. They were housed in stainless steel cages at room temperature (28-32°C) and under natural dark cycle. The animals were fed with standard commercial pellets [17]. Five days prior to commencement of the experiment, rats were randomly divided into 6 groups of 5 rats per treatment group such that the weight differences within and between treatment groups to not exceed ±20% of the average weight of the rat population [16]. Prior authorization for the use of laboratory animal was obtain from the Cameroon National Ethics Committee (Ref. N° FWIRB 00001954).
2.4. Induction and Treatment of Hyperglycemia
- Distilled water (10 mL/kg), A. boonei (200 and 500 mg/kg), glibenclamide (5 mg/kg) and metformin (20 mg/kg) were single, daily oral administered for 30 days between 07:00-8:00 a.m. 30 minutes after pretreatment, all rats in groups 2 to 6 were intra-peritoneously injected with dexamethasone at 0.4 mg/kg (Ogawa et al., 1992) as follow:Group 1 (normoglycemic control group): Distilled water (10 mL/kg, p.o.) + normal saline (1mL/kg, i.p.). Group 2 (hyperglycemic model control group): Distilled water (10 mL/kg, p.o.) + Dexamethasone (0.4 mg/kg, i.p.)Group 3 (experimental group): A. boonei (200 mg/kg, p.o.) + Dexamethasone (0.4 mg/kg, i.p.)Group 4 (experimental group): A. boonei (500 mg/kg, p.o.) + Dexamethasone (0.4 mg/kg, i.p.)Group 5 (reference group): Glibenclamide (5 mg/kg, p.o.) + Dexamethasone (0.4 mg/kg, i.p.)Group 6 (reference group): Metformin (20 mg/kg, p.o.) + Dexamethasone (0.4 mg/kg, i.p.). Before administration to animals, lyophilized extract (200 and 500 mg) was recovered with distilled water, for a total volume of 10 mL.
2.5. Oral Glucose Tolerance Test (OGTT) in Normal Rats
- The anti-hyperglycemic effect of the aqueous extract of A. boonei was studied at 50, 100, 200 and 500 mg/kg. The animals were fasted for 16 h and treated with the aqueous extract of A. boonei and glibenclamide. Control rats were treated with the vehicle. At 0 min, blood samples were collected from the tip of tail. Then the animals were treated with the aqueous extract of A. boonei dissolved in the vehicle (distilled water), the normal control rats were treated with the vehicle alone. Glibenclamide (5mg/kg) was used as positive control. All the treatments were given orally. 30 min after the treatment, all groups received glucose (3 g/kg bw) orally and the blood glucose concentrations were determined from the tip of a tail before drug administration, and at 30, 60, 90, 120 and 180 min thereafter.
2.6. Sub-chronic Antidiabetic Effect of A. boonei
- For subchronic test, experimental rats were treated during 30 days. A. boonei (200 and 500 mg/kg), metformin (20 mg/kg), glibenclamide (5 mg/kg) as well as vehicle were administrated. Body weight and blood glucose were monitored every weeks. The relative weight of internal organ was calculated as follow:
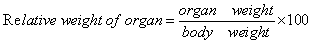 | (1) |
2.7. Biochemical Analysis and Estimation of Liver and Kidney Antioxidant Parameters
- Biochemical analysis was determined according to previous methods [18]. The rats were sacrified by cervical dislocation and arteriovenous blood was collected. Serum was then collected from the clotted blood at room temperature by centrifugation at 3000g for 10 min. Lipid profile (cholesterol and triglyceride), urea, bilirubin, total protein were estimated using diagnostic kits, Fortress, UK, transaminases (ALT, AST) activities were determined using the method of Reitman and Frankel [19], creatinine was also estimated using Bartels et al. [20] procedure. After blood collection, the liver, kidney, lung and heart were quickly removed and weighed. The liver and kidney were used for preparation of homogenate (20%). The supernatant was stored at -20°C for antioxidant parameters. Wilbur et al. [21] method was used to determined lipid peroxidation by measuring Malondialdehyde (MDA), reduced glutathione (GSH) and superoxide dismutase (SOD) were determined using the method of Ellman [22] and Misra and Fridovich [23], respectively. Catalase (CAT) activity was assayed using the method of Haldar et al. [24].
2.8. Acute Oral Toxicity Test
- Acute oral toxicity study was conducted according to the guidelines on acute oral toxicity test n° 423 [25]. Twenty-four Wistar rats fasted overnight, but allowed free access to water were randomly divided into 3 groups of 8 rats (4 males and 4 females). Control group received distilled water and 2 groups received the extract at the doses of 2000 mg/kg and 5000 mg/kg. The rats were not fed for 3 h following administration. Eventual signs of toxic effects or mortality were observed 3 h after administration then, for next 48 h. Rats were kept for observation and monitoring of food and water intakes for 14 days.
2.9. Data Analysis
- The data are presented as mean ± SEM from five animals in each group. All data were statistically analyzed using one-way analysis of variance (ANOVA) followed by post-hoc test (Student-Newman-Keuls) using ‘‘Graph Pad Prism’’ software. A value of p<0.05 was considered statistically significant.
3. Results
3.1. Oral Glucose Tolerance Test (OGTT)
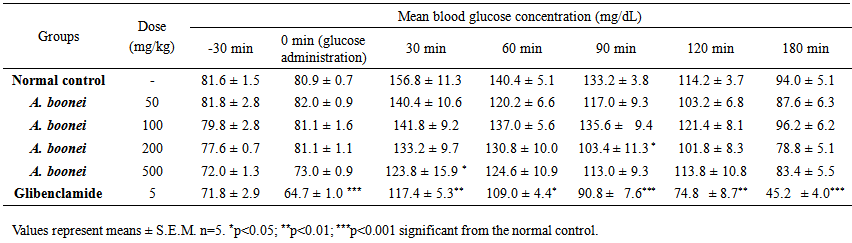
- In the acute test, glucose load increased glycemia in normal control group. Rats blood glucose values in this group varied from 81.6 ± 1.56 to 156.8 ± 11.33 mg/dL at 30 min after glucose administration and return to normal (94.0 ± 5.11 mg/dL) at 180 min (Table 1). The treatment with aqueous extract of A. boonei at 500 mg/kg reduced significantly (p<0.05) the plasma glucose levels when compared to the normal rats at 30 min. The same significant reduction where obtained with the dose of 200 mg/kg at 90 min (Table 1). Glibenclamide significantly prevented the rise in blood glucose throughout the experiment.
 | Table 1. Effect of Alstonia boonei aqueous extract on oral glucose tolerance test (OGTT) in normal rats |
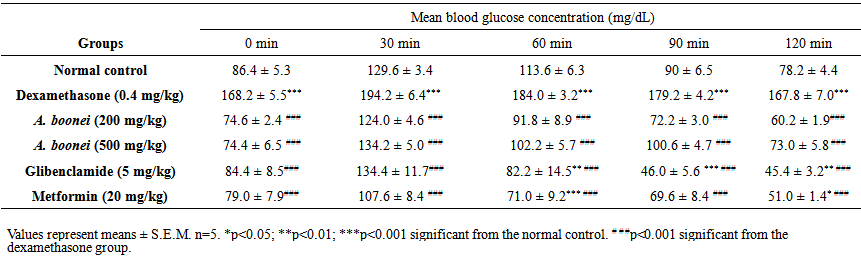 | Table 2. Effect of Alstonia boonei aqueous extract on oral glucose tolerance test (OGTT) in dexamethasone-induced-hyperglycemic rats after 4 weeks of treatment |
3.2. Fasting Blood Glucose Level
- Table 3 shows the effects of the aqueous extract of A. boonei on fasting blood glucose level in dexamethasone- induced-hyperglycemic rats. The negative control shows a significant and gradual increase in fasting blood glucose levels during the treatment period. The aqueous extract treated animals showed a highly significant reduction (p<0.001) in plasma glucose levels when compared to dexamethasone group. The maximum reduction (55.76%) was obtained with A. boonei (500 mg/kg) at week 4.
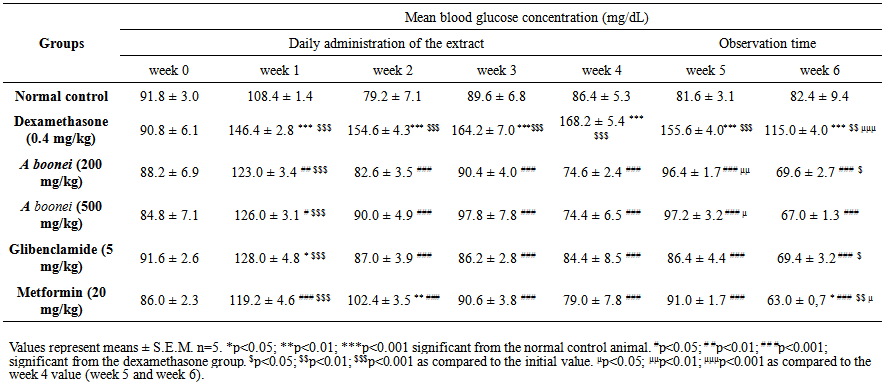 | Table 3. Sub-chronic effects of the aqueous extract from Alstonia boonei on fasting blood glucose level in dexamethasone-induced-hyperglycemic rats |
3.3. Body Weight and Weight of Internal Organs
- Table 4 shows the effect of the aqueous extract of A. boonei on body weights in dexamethasone- induced-hyperglycemic rats. A significant decrease (p<0.001) of body weight was observed to the negative control group when compared to the normal control group at week 4. A statistically significant (p<0.05) and (p < 0.01) increase in body weight of positive control groups glibenclamide and metformin respectively was observed as compared to the normal group receiving only vehicles for 4 weeks.
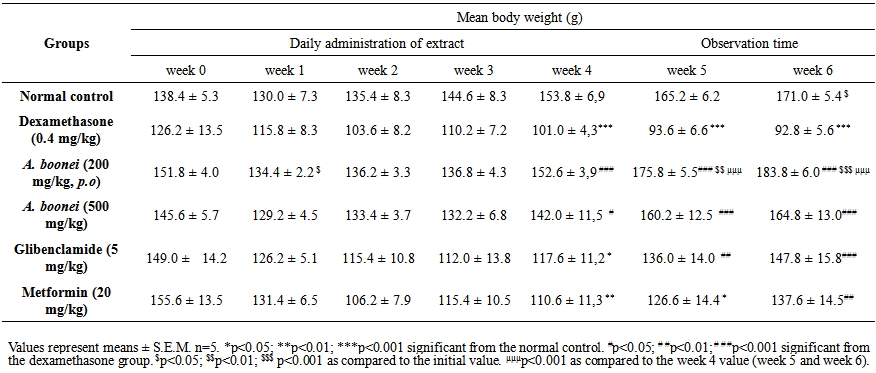 | Table 4. Sub-chronic effects of the aqueous extract from Alstonia boonei on body weights in dexamethasone-induced-hyperglycemic rats |
- The body weight of the six groups of rats all displayed decreasing tendency from the initial values (week 0) to the first week, compared to the initial values respectively. From the first week to the fourth week, the body weight of all groups except the negative control group displayed increasing tendency. At end of experimentation (week 6), only control group and A. boonei at 200 mg/kg had a significant increase (p<0.05) and (p<0.001) respectively in body weight when compared with the initial value (Table 4).After administration of A. boonei aqueous extract and chemicals for 4 weeks, there was no significant difference in relative weight of internal organs when compared with normal control (Table 5). However, a negative control group there was a significant increased in liver, kidney and hearth relative weight when compared with normal control group (Table 5).
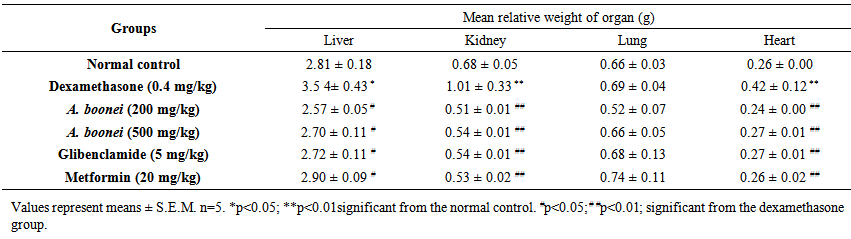 | Table 5. Liver, kidney, lung and heart weight of dexamethasone-induced-hyperglycemic rats after 4 weeks treatment with Alstonia boonei aqueous extract |
3.4. Estimation of Serum Biochemical Parameters
- In dexamethasone-induced-hyperglycemia control group biochemical parameters like triglycerides, total cholesterol, ALT, AST, bilirubin, creatinine and urea were significantly (p<0.001) elevated, and total protein significantly (p<0.001) decreased as compared to normal control group (Table 6). Concomitant, treatment with the aqueous extract of A. boonei (200 and 500 mg/kg) and dexamethasone significantly reduced (p<0.001) triglycerides, total cholesterol (p<0.01), AST and bilirubin (p<0.001), creatinine (p<0.01), urea (p<0.001), when compared to dexamethasone-induced-hyperglycemia control group. A. boonei reduced significantly (p<0.001) ALT only at dose of 500 mg/kg, but at the two doses (200 and 500 mg/kg), aqueous extract of A. boonei increased significantly (p<0.01) total protein when compared to the dexamethasone- induced-hyperglycemia control group.
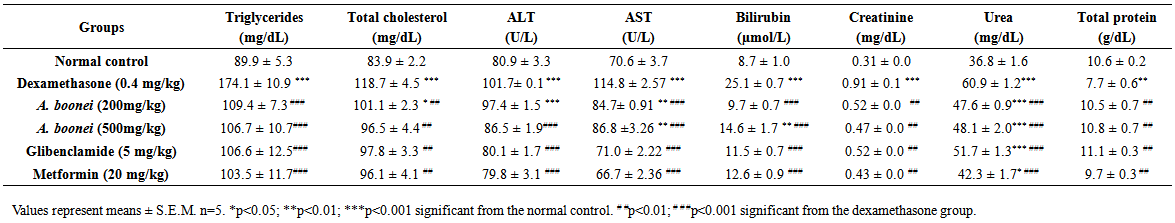 | Table 6. Effect of Alstonia boonei aqueous extract on serum biochemical parameters in dexamethasone-induced-hyperglycemic rats after 4 weeks treatment |
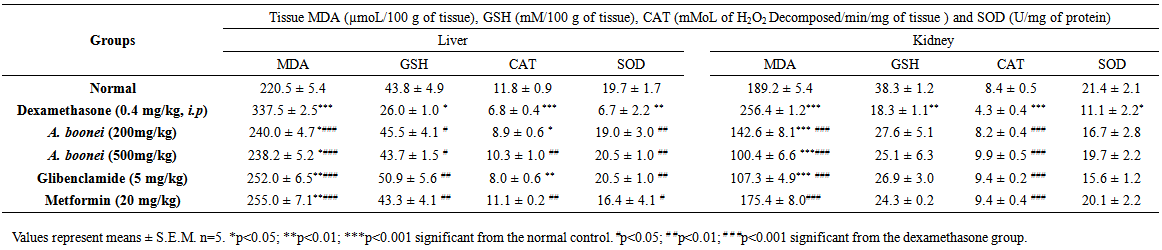
3.5. Estimation of Tissue Antioxidant Parameters
- The level of MDA, GSH, CAT and SOD activities in liver and kidney of experimental hyperglycemic rats are shown in Table 7. There was a highly significant elevation (p<0.001) of MDA in liver and kidney in negative control group, when compared to the normal control group. GSH, SOD and CAT were decrease (p<0.05, p<0.01 and p<0.001 respectively) in liver of dexamethasone rats compared with normal rats. In kidney, SOD, GSH and CAT were decrease (p<0.05, p<0.01 and p<0.001 respectively) when compared to normal rats. The aqueous extract treated animals showed a higher significant decrease (p<0.001) of MDA in liver and kidney when compared to the dexamethasone group. The aqueous extract treated animals also showed significant increase (p<0.05) of GSH only in liver at the two doses when compared to dexamethasone rats. CAT increased significantly (p<0.01) in the liver of aqueous extract rats only at the dose of 500 mg/kg but, there was highly increased (p<0.001) in the kidney at two doses (200mg/kg and 500 mg/kg) when compared to the dexamethasone rats. There was significant elevation (p<0.01) of SOD only in liver at the two doses of the aqueous extract.
3.6. Acute Oral Toxicity
- Alstonia boonei aqueous extract at the doses of 2000 mg/kg or 5000 mg/kg sowed no toxicity signs. All animal included in the test were healthy after the 14 days observation period. The macroscopic pathology observations performed after sacrifice on the 15th day, revealed no visible lesions in any animals. The LD50 value of Alstonia boonei was considered greater than 5000 mg/kg.
4. Discussion
- The present study reports for the first time the effect of the aqueous extract of A. boonei stem bark as anti-diabetic and antioxidant agent. In this study, hyperglycemia and dyslipidemia were induced in rats by repeated intraperitoneal injection of dexamethasone (0.4 mg/kg/day). These dexamethasone-induced derangements in blood glucose and lipids were attenuated by daily oral pretreatment with 200 and 500 mg/kg of aqueous extract of A. boonei. In the present study, A. boonei significantly prevented the rise in blood glucose induced by dexamethasone throughout the experimental period. These results strongly suggest that A. boonei aqueous extract possessed antihyperglycemic properties. This may be related to the stimulation of insulin secretion and or glucose uptake in muscle and adipose tissue. This antihyperglycemic property was confirmed with OGTT test. In fact, A. boonei at all the doses used significantly prevented the rise of glycemia after glucose load. The result obtained were similar to those of glibenclamide and metformin, standard oral antidiabetic drug. Glibenclamide is shown to stimulate insulin released by pancreatic β-cells. A. boonei could similarly stimulate the insulin released. This hypothesis is strengthen by the result of Akinloye et al. [15] who reported a significant reduction in blood glucose level in STZ-induced diabetic mice when treated with A. boonei stem bark extract. This author also suggested that, hypoglycemic effect of the A. boonei stem bark in mice could be attributed to the reduction in the activity of the regulatory hepatic glucogenic enzymes namely glucose-6-phosphate and fructose-1.6-biphosphate. It could be inferred that glucose synthesis would be decrease, thus, reducing the blood glucose level. Our results are also similar to those of Bandawane et al. [14] who reported the antidiabetic and antihyperlipidemic effect of Alstonia scholaris, a specie in the same genera in streptozotocin induced type 1 diabetes.Induction of hyperglycemia with dexamethasone accelerated fatty acid oxidation, aggravate dyslipidemia and liver steatosis [26, 27]. Rats treated both with A. boonei aqueous extract and dexamethasone showed a significant improvement in body weight as compared to dexamethasone-induced-hyperglycemia control animals; hence aqueous extract of A. boonei exhibited a marked effect in controlling the loss of body weight of dexamethasone- induced-hyperglycemia control rats.The treatment with this extract reduced the total cholesterol and triglyceride levels when compared to the negative control rats. This suggests that the mode of action of this extract affect lipid metabolism probably by affecting lipoprotein lipase.In the aqueous extract treated dexamethasone- induced-hyperglycemia rats the reduction in urea level indicated reduced proteolysis [28] and this might be the reason for the increase in the body weight of the animals. The relative weight of kidneys increased in dexamethasone- induced-hyperglycemia control rats, suggesting at least a pre-diabetic state [29]. Our results are in line with those of Raju et al. [30] who reported that weight of kidneys increases in diabetic condition.Administration of dexamethasone induced a significant increase of AST, ALT, bilirubin, creatinin and urea. Administration of the plant extract significantly prevented the rise of those parameters suggesting a protective of effect the plant extract in liver and kidney function (Table 6).Oxidative stress in diabetes mellitus has been show to coexist with impairment in the endogenous antioxidant status and removal of oxidative damage molecules by activating antioxidant enzymes such as CAT, gluthathione S-transferase (GST), gluthathion peroxidase (GPx), etc. These antioxidants are able to resist oxidative stress by scavenging free radicals, inhibiting lipid peroxidation and increasing GSH and CAT activity [31]. Treatment with the aqueous extract of A. boonei elevated the level of GSH thus protecting the liver and kidney from oxidative stress induced by dexamethasone. It was found that catalase activity was decreased in dexamethasone-induced-hyperglycemic rats. Administration of aqueous extract of A. boonei in dexamethasone-induced-hyperglycemic rats showed normalization of CAT, preventing oxidative injury of the liver and kidney. SOD activity was significantly reduced in dexamethasone-induced-hyperglycemic control rats, whereas treatment during four weeks with plant extract provided depletion against activity of this enzyme in the liver.The present study revealed that single administration of A. boonei aqueous extract showed no sign of toxicity and that the LD50 value was greater than 5000 mg/kg, suggesting a very low toxicity of our extract. However, our results are in contradiction with those of Olufunsho et al. who reported a LD50 value of 4168.69 mg/kg in mice [32]. This difference could be explained by the type of animal used and by the bioactive compound in the extract.
5. Conclusions
- In conclusion, our study show that A. boonei posses antihyperglycemic and antioxidant activities therefore, adds credence to the traditional use of the aqueous extract of A. boonei stem bark by the indigenous Cameroonians tribe to treat diabetes.
Conflict of Interest Statement
- We declare that we have no conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML