-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2014; 3(2): 22-26
doi:10.5923/j.diabetes.20140302.03
Dual Energy X-Ray Absorptiometry Quantification of Visceral Adipose Tissue
1Thomas Jefferson University
2Charter School of Wilmington
Correspondence to: Xia Bi, Thomas Jefferson University.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abdominal obesity, especially the visceral component of adipose tissue (VAT), is strongly associated with metabolic and cardiovascular risk in humans. The differences in gender and ethnicity with regard to body composition and metabolic risk have also been correlated with higher VAT risk. For example, Asians accumulate more VAT despite similar BMI and waist circumference measurements, whereas African-Americans have a lower VAT yet a higher risk for metabolic disease. Computed tomography (CT) and magnetic resonance imaging (MRI) scanning have been used extensively to measure the type and distribution of body fat, and have contributed greatly to the understanding of various body composition phenotypes in obesity-related complications. Despite the recent advances in imaging modalities to visualize abdominal fat, each has its limitations. CT exposes individuals to large doses of ionizing radiation; MRI requires longer imaging times and sophisticated post-imaging processing. Dual energy X-ray absorptiometry (DEXA) can accurately and noninvasively measure body composition with low X-ray exposure and a short-scanning time. This review summarizes current progress on using DEXA as an emergent imaging modality to measure VAT depot and define health risks associated with insulin resistance and metabolic disease. With the new fully automated analysis software for DEXA, it is now possible to differentiate types of fat and directly measure VAT to characterize body composition and predict metabolic risk. In conclusion, we comment on establishing DEXA as an effective and economical tool in the clinic to identify at-risk individuals and assess their cardiometabolic risk associated with depot-specific adiposity.
Keywords: Obesity X-ray diabetes
Cite this paper: Xia Bi, Sophia Ho, Dual Energy X-Ray Absorptiometry Quantification of Visceral Adipose Tissue, International Journal of Diabetes Research, Vol. 3 No. 2, 2014, pp. 22-26. doi: 10.5923/j.diabetes.20140302.03.
Article Outline
1. Introduction
- Obesity is an epidemic affecting approximately one in three adults (35.7%) and one in six children (17%) in the United States today [1]. It is a major cause of death due to the comorbidities related to tissue inflammation and insulin resistance that precipitate a dramatically increased risk for multiple health problems including hypertension, diabetes, and cardiovascular disease [2]. However, not all body fat contributes equally to cardiometabolic risk. Recent research has come to recognize the clinical significance of various types and distributions of fat, and has arrived at the consensus that abdominal adiposity is most strongly associated with cardiovascular and metabolic risk in humans [3-5]. Other etiological factors including gender, genotype, ethnicity, and family history also help to mediate the variation in abdominal fat accumulation, and therefore, need to be considered when assessing risk.
2. The Role of Abdominal Visceral Fat in Phenotypes
- Within the abdominal fat depot, visceral adipose tissue (VAT) rather than subcutaneous adipose tissue (SAT), is considered to be the compartment most associated with adverse outcomes such as diabetes, metabolic syndrome, and cardiovascular disease [6]. Research has shown that VAT releases large amounts of free fatty acids, hormones, and inflammatory cytokines that interact with hepatocytes and various immune cells in the liver, promoting an insulin-resistant state [7]. Consequently, the tendency to accumulate VAT is a key correlate and perhaps driver of obesity-related complications such as dyslipidemia, insulin resistance and type 2 diabetes.More recent studies have also demonstrated gender and race-ethnic differences in body composition and the metabolic aberrations associated with abdominal obesity [8]. For instance, men tend to have higher VAT than pre-menopausal women, which may explain why men are at greater risk for metabolic complications associated with obesity [9]. One study of 62 African American and 98 Caucasian youth showed that African American boys had higher SAT and lower VAT than Caucasian boys, wherein the magnitude of the difference is increased with increasing waist circumference (WC) [10]. People of South Asian descent, who are more susceptible to metabolic and cardiovascular disease, also showed a greater proportion of VAT for a given total body fat when compared with Europeans [11].While these studies support the role of VAT in different body phenotypes, more data is needed to elucidate gender and racial differences in the relationship between VAT and metabolic risk factors. Despite having a more favorable lipid profile and a lower VAT than Europeans, African-Americans are at an increased risk for type 2 diabetes and metabolic syndrome [8, 12]. This may be due to similar concentrations of inflammatory biomarkers, particularly IL-6 and fibrinogen [13], which highlight the importance of other factors that contribute to obesity-related complications. Studies have also suggested that the deep layer of SAT, which is separated from the superficial layer by fascia superfialis, may be the major predictor of adverse events [14]. In addition, there is inconsistentdata that compares VAT with other anthropometric and indexes of adiposity, and more studies are needed to explicate the association of VAT with individual metabolic risk factors, particularly in people of different age, gender, and ethnicities.
3. Measuring Abdominal Visceral Fat
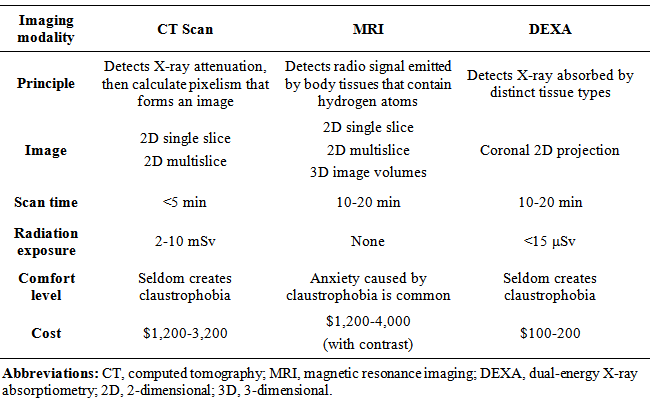
- Clinically, measurement of WC is a proxy for more direct measurement of VAT. Some studies have shown WC to be a better estimate of VAT than other anthropometric measures such as body mass index (BMI), hip circumference (HC), abdominal sagittal diameter (ASD), and waist-hip-ratio (WHR) [15], while others showed that waist-height-ratio (WHtR) may be superior [16]. Although these measures are useful to screen patients at high risk for diabetes and metabolic syndrome, they are often unreliable and inconsistent in the clinical setting. For instance, WC is unable to assess the amount, type (SAT vs. VAT) and distribution of adipose tissue, and there exists slight variation at the anatomic level at which physicians measure WC at each visit. One study showed that WC measured below the lowest rib correlated most strongly with VAT, whereas WC measured above the iliac crest had the lowest associations with VAT and cardiometabolic risk factors in women [17]. In addition, there exist gender and race-ethnic variations in using anthropometric indexes to assess the accumulation of VAT and its associated risk. Researchers have advocated lowering cut-off values of the risk factors used to identify and intervene individuals at high risk for metabolic syndrome (MetS) in South Asians due to its high prevalence in this particular population [18]. This trend of higher rates of diabetes and MetS remains high even among US Asian Indians, necessitating earlier intervention in this rapidly growing ethnic group in the US [19]. Due to limitations in using anthropometric indexes to estimate metabolic risk, imaging techniques such as X-ray computed tomography (CT) and magnetic resonance imaging (MRI) have been considered the “gold standards” in the measurement of type and distribution of body fat, and have contributed greatly to the understanding of various body composition phenotypes and race-ethnic variability in susceptibility to metabolic disease [20, 21]. However, CT exposes patients to large doses of radiation and requires manual image analysis to estimate VAT. MRI is expensive and requiresa highly skilled technician as well as sophisticated post-imaging processing. These issues have limited their clinical use as a screening tool for VAT, imposing a great need for more economical and accessible method in assessing metabolic risk. Table 1 lists the key characteristics of the three most commonly used imaging techniques (ie. CT, MRI, and DEXA) used to quantify body composition.
|
4. Characterizing Abdominal Visceral Fat Using DEXA
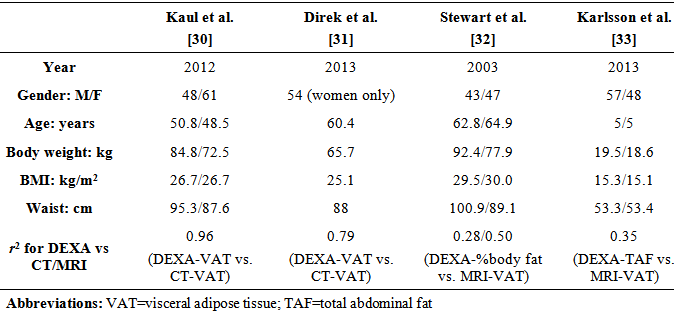
- Dual energy X-ray absorptiometry (DEXA), which was originally developed to examine whole body and regional bone mineral density, can accurately measure body composition while only exposing subjects to a minimal dose of radiation and a short scanning time [22]. DEXA measures whole body composition as 2-dimensional projections and segregates soft tissue into lean and fat compartments based on regional differences in X-ray absorption. During the scan, the subject lies supine on the table, and an X-ray generator emits two energy levels of pencil beam beneath the table that is absorbed by an X-ray detector above the table. The two energy levels are each absorbed by soft and dense tissue, thereby allowing the quantification of whole and regional fat and fat-free mass in the subject. The entire process takes approximately 10 to 20 minutes.DEXA have been used in the evaluation of overall and regional assessments of body composition in healthy individuals as well as patients with chronic metabolic diseases [23]. There exists strong correlations between DEXA and MRI whole body composition, with coefficients of variation of less than 2% for DEXA-derived adiposity measures [24]. However, researchers need to manually define their margin of interest to eliminate as much of the SAT as possible, which renders DEXA-derived VAT highly operator-dependent. Park et al showed that DEXA measured at the L2-4 and L2-upper iliac were best associated with total VAT as well as MRI-derived VAT area at L4-5 in 90 non-obese healthy men [25].A limitation of using DEXA to measure total abdominal fat in previous studies was the inability to distinguish between SAT and VAT, and therefore showed DEXA to be inferior to anthropometric and other imaging modalities in estimating VAT. In a cross sectional study on 76 Caucasian adults, DEXA trunk fat and abdominal fat appeared to be slightly better predictors of total abdominal fat, but not the visceral component, when compared to WC and ASD [26]. One study in an elderly population was unable to show an advantage of DEXA over other measures in predicting visceral fat [27]. In another study, DEXA was only equivalent to WC and WHtR in predicting VAT in non-obese men [28]. When compared to MRI scanning, Scherzer et al saw an increasing difference between DEXA- and MRI-measured adipose tissue depots as average fat increases in 877 HIV+ and 260 healthy subjects (p< 0.001) [29]. This suggests that DEXA may be more accurate in predicting VAT in non-obese subjects but may overestimate adiposity in the obese population.A newer software available on some DEXA scanners now uses a fully automated method to estimate VAT by segmenting abdominal fat into SAT and VAT within the android region, which is defined by 20% of the distance from the top of iliac crest to the base of skull [30].VAT was obtained via empirically derived geometric constants to estimate SAT in the android region derived VAT, then subtracting SAT from the total abdominal fat. This method has been validated against CT in a patient population with a wide range of BMI (18.5-40kg/m2) [30]. A table comparing basic characteristics and correlation coefficient between DEXA-derived body composition variables and CT- or MRI- derived VAT by several different authors is shown below (Table 2). Of note is that Kaul et al. and Direk et al. were able to directly estimate VAT and arrived at a stronger correlation coefficient between DEXA- and CT-derived VAT (0.96, 0.79, respectively), highlighting the strong agreement between these two imaging techniques. In comparison, Stewart et al. and Karksson et al. used traditional DEXA to image body composition, and obtained lower correlation coefficient between DEXA-derived body composition and MRI-derived VAT (0.28/0.50, 0.35, respectively). This points to the possibility of using DEXA as an emergent imaging modality to predict VAT-associated metabolic aberrations of at-risk individuals in the clinical setting.
|
5. Conclusions
- Obesity-related complications are largely mediated by abdominal visceral fat as it constantly releases free fatty acids, chemokines, and cytokines into the portal circulation to promote a state of inflammation and insulin resistance. VAT has been recognized as a risk factor for metabolic syndrome, diabetes, and cardiovascular disease. The exact reason for the propensity of VAT to be associated with metabolic and endocrine perturbations remains speculative, but compared to SAT, VAT is more metabolically active with a greater innervation, cellularity and blood flow. VAT is also more resistant to insulin, and has a greater capacity to take up glucose and generate free fatty acids than SAT, which exert profound effects on adipose tissue accumulation and metabolism. The advent of DEXA has allowed for rapid and non-invasive measurement of various body compositions and direct estimation of VAT using the most updated software analysis. In the clinical management of patients at high risk for obesity-related complications, whole body DEXA may replace CT and MRI as a more reliable and more accessible tool to provide information about depot-specific adiposities and their predisposition to metabolic and cardiovascular disease.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML