-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2013; 2(6): 101-111
doi:10.5923/j.diabetes.20130206.03
Effect of Ghrelin on Testicular Functions in Streptozotocin Induced Type 1 Diabetic Rats
Maher N. Ibrahim, Ali Kh. Asalah, Dalia I. Abd-Alaleem, Suzan M. M. Moursi
Physiology department-Faculty of medicine-Zagazig University
Correspondence to: Ali Kh. Asalah, Physiology department-Faculty of medicine-Zagazig University.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Background:Oxidative stress is increased inDiabetes mellitus (DM). Ghrelin is a peptide hormone produced by different tissues and its circulating level is decreased in type 1 DM. Recently, it has been shown to have antioxidant properties.Objective: This study is designed to investigate the effects of diabetes mellitus on testicular functions and the role of ghrelin in the modulation of these effects in a rat model of type 1 diabetes mellitus induced by Streptozotocin.Materials and methods: 32 healthy adult albino male rats of 170-195 gm initial body weight were utilized. The Rats were randomly and equally divided into 4 groups, group (1a): Vehicle treated (control) group, group (1b): Ghrelin treated normal group, group (IIa): STZ-diabetic group and group (IIb): Ghrelin treated diabetic group. Rats were weighed and checked for the serum levels of glucose, insulin, FSH, LH& testosterone levels, epididymal sperm count and motility, testicular malondialdhyde (MDA) level and superoxide dismutase (SOD), catalase (CAT) & glutathione peroxidase (GPX) activities and testicular weight & histopathology.Results and discussion: STZ-induced diabetes significantly decreased body weight, testes weight, serum insulin, FSH, LH & testosterone levels, epididymal sperm count & motility and testicular SOD, CAT & GPX activities. However, they significantly increased serum glucose & testicular malondialdhyde levels parallel with deterioration of the testicular histoarchitecture. In addition, it was found that exogenous administration of ghrelin resulted in a significant recovery of the above mentioned parameters in the diabetic group, while in the normal group, it was only associated with potentiating of the testicular antioxidant system as well as a significant increase in the body and testes weights. Conclusion: Ghrelin has a potential protective role against diabetes-induced pituitary testicular dysfunction which may be due to its antioxidant properties and maintenance of glucose and insulin homeostasis.
Keywords: Diabetes, Ghrelin, Testicular Function, Oxidative Stress
Cite this paper: Maher N. Ibrahim, Ali Kh. Asalah, Dalia I. Abd-Alaleem, Suzan M. M. Moursi, Effect of Ghrelin on Testicular Functions in Streptozotocin Induced Type 1 Diabetic Rats, International Journal of Diabetes Research, Vol. 2 No. 6, 2013, pp. 101-111. doi: 10.5923/j.diabetes.20130206.03.
1. Introduction
- DM is the most common chronic metabolic disorder that characterized by hyperglycemia. It is caused by abnormal insulin production, insulin resistance or often both[1,2]. Diabetic patients generally experience sexual abnormalities such as sexual dysfunction, impotence and infertility[3, 4]. There are apparently contradictory reports concerning male reproductive parameters in diabetics. Some investigators detected low serum levels of gonadotropins and testosterone as well as a decrease in sperm count and motility[5, 6, 7] howevers, others reported elevated or unaffected pituitary gonadotrophins and testosterone but with a reduction in their receptors[8]. Moreover, other studies reported that diabetic men may represent normal semen parameters, but they have a higher level of damage in sperm nuclear and mitochondrial DNAs[9]. In contrast, other study showed that sperm count and concentration were significantly increased in type 1 diabetic subjects while semen volume and sperm motility were significantly decreased[10]. It is indicated that the oxidative stress is more in hyperglycemic state due to the excessive production of reactive oxygen species (ROS) and decreased efficiency of anti-oxidant enzyme defenses[11, 12]. Stability and capacity of the antioxidant defense against ROS during chronic diabetes plays an important role in the outcome of long term complications caused by ROS[13]. So, antioxidants can be useful in the treatment of male infertility as reported by many researchers[12, 14, 15, 16].Ghrelin is a 28-amino-acid peptide initially isolated from human and rat stomach as an endogenous ligand for the growth hormone secretagogue receptor (GHS-R) which acts on the pituitary to stimulate GH release and on the hypothalamus to enhance food intake[17, 18]. Its circulating plasma level is 100–150 fmol/ml[19] which is reduced in type 1 DM[20]. Ghrelin secreting cells have also been identified in pancreas[21], hypothalamus[22], pituitary[23], interstitial Leydig cells and in Sertoli cells[24).Ghrelin is involved in glucose metabolism, whatever, data regarding ghrelin influence on insulin secretion are still contradictory; both inhibitory[25] and stimulatory effects having been reported[26, 27]. Furthermore, it has been proposed that ghrelin is an endogenous antioxidant and functions as a free radical scavenger. The antioxidative properties of ghrelin via increasing of antioxidant enzyme activities and reduction in lipid peroxidation have been reported in preadipocyte cell lines[28], rat ovary[29], liver [30] & gastric injuries[31] and sensorimotor neuropathy[32]. Moreover, ghrelin treatment protected against testicular germ cells damage induced by oxidative stress of heat exposure and ischemia reperfusion injury, and this effect may be due to its antioxidant properties[33,34].This study is designed to investigate the effects of diabetes mellitus on testicular functions and evaluate the effects of exogenously administered ghrelin in the modulation of these effects in rat model of type 1 diabetes mellitus induced by Streptozotocin treatment. The level of androgenic hormones, sperm quality, testicular histoarchitecture & antioxidant enzyme activities were examined in trial to clarify possible involved mechanisms.
2. Material and Methods
- This study was conducted on 32 healthy adult albino male rats of170-195 gm weight. They were collected from the animal house of Faculty of Veterinary Medicine- Zagazig University. Rats were kept in steel wire cages (4/cage) in the physiology animal house in Faculty of Medicine -Zagazig University under hygienic conditions. They had free access to water, kept at room temperature and were maintained on 12 h light/dark cycle. Rats were adapted to the new environment for one week before the experiment going on. All investigations were conducted in accordance with the guiding principles for the care and use of research animals and were approved by the Institutional Research Board of Faculty of Medicine -Zagazig University. Rats were randomly divided into four equal groups, group (1a): Vehicle treated (control) group, each rat received every other day for 30 days a single s.c injection of 100 μl saline, group (1b): Ghrelin treated normal group, each rat received every other day for 30 days a single s.c injection of ghrelin (Acylated powder form, Sigma Aldrich Co.-USA) at a dose of 2 nmol/100 μl saline[33], group (IIa): STZ-diabetic group, in which experimental diabetes was induced by an i.p injection of freshly prepared streptozotocin (Sigma Aldrich Co.-USA) at a dose of 60 mg/kg dissolved in saline[35] in overnight fast rats. After 72 hours, fasting glucose level was measured using glucometer (ACCU CHEK, Rhoche Diagnostics, Germany) and rats with blood glucose levels above 360 mg/dl were included for this study[8] with weekly monitoring of those levels throughout the study. In this group each rat received every other day for 30 days[16] a single s.c injection of 100 μl saline and group (IIb): Ghrelin treated diabetic group, each rat received every other day for 30 days a single s.c injection of ghrelin at a dose of 2 nmol/100 μl saline[33].The dose of ghrelin used in the experiments was comparable with the amounts of ghrelin secreted into the blood during starvation: exogenous administration of 1 nmol of ghrelin is able to induce a significant elevation (2.4 to 2.6-fold increase) in serum levels of total ghrelin 1 h after injection[36], whose magnitude is in the range of that induced by fasting[37]. Sample collection: Blood samples were collected from retro-orbital venous plexus 24 hours after the last injection of ghrelin and serum was separated by centrifugation of blood at 3000 rpm for 20 minutes and kept deep frozen at (-20℃) until used to measure serum glucose, insulin, FSH, LH and testosterone levels. After collecting blood samples, laparotomy was conducted after the animals were sacrificed by cervical dislocation under mild ether anesthesia. Testes and epididymes were collected. The epididymes were used for the evaluation of sperm parameters. The testes were weighed then the right one was processed for histopathological studies and the left one was homogenised for biochemical estimations of MDA levels and SOD, GPX & CAT activities. Preparation of testis homogenate: The testes were sliced and homogenized in cold 50 mM phosphate buffer (pH 7.0) containing 0.1 mM EDTA to give 10% homogenate (w/v). The homogenates were centrifuged at 1000 r.p.m. for 10 min. The supernatants were separated and used for enzymes activity assays and lipid peroxidation determination[38]. Biochemical Analysis1) Serum glucose level: according to Tietz,[39] using glucose enzymatic (GOD-PAP) - liquizyme Kits(Biotechnology, Egypt) was measured by using spectrophotometer (spectronic 3000 Array, Germany) at 546 nm.2) Serum insulin level: according to Temple et al.[40] using KAP1251-INS-EASIA (Enzyme Amplified Sensitivity Immunoassay) Kits (BioSource Europe S.A., Belgium), was measured by using spectrophotometer (spectronic 3000 Array, Germany) at 450 nm.3) Serum FSH level: according to Rebar et al.[41] using Follicle-Stimulating Hormone (FSH) enzyme immunoassay test kit (Catalog Number: BC-1029, BioCheck, Inc 323 Vintage Park Dr. Foster City, CA 94404), measured by using spectrophotometer (spectronic 3000 Array, Germany) at 450 nm4) Serum LH level: according to Tietz,[39] using Luteinizing hormone (LH) enzyme immunoassay test kit (Catalog Number: BC-1031, BioCheck, Inc 323 Vintage Park Dr. Foster City, CA 94404), was measured by using spectrophotometer (spectronic 3000 Array, Germany) at 450 nm5) Serum testostosterone level: according to Tietz,[39] using testosterone enzyme immunoassay test kit (Catalog Number: BC-1115, BioCheck, Inc 323 Vintage Park Dr. Foster City, CA 94404), was measured by using spectrophotometer (spectronic 3000 Array, Germany) at 450 nm6) Testicular antioxidant system evaluation:ο Assay of superoxide dismutase (SOD) activity: according to the method described by Kakkar et al.[42], by using spectrophotometer (spectronic 3000 Array, Germany) at 560nm.ο Assay of of catalase (CAT) activity: according to the method described by Luck[43], by usingspectrophotometer (spectronic 3000 Array, Germany) at 240nm.ο Assay of glutathione peroxidase (GPX) activity: according to the method described by Reddy et al.[44], by using spectrophotometer (spectronic 3000 Array, Germany) at 430nm.ο Assay of MDA level: according to the method described by Ohkawa et al.[45], by usingspectrophotometer (spectronic 3000 Array, Germany) at 535nm.Sperm parameters analysis: The right epididymis of each rat was dissected, removed and minced in 2 ml of Hank’s buffer salt solution (HBSS) at 37°C[8]. After 5 min incubation at 37°C, the caudal epididymis sperm was determined using the standard hemocytometric method[46]. The percentage of sperm motility was calculated using the number of live sperm cells over the total number of sperm cells[16].Histopathological examination:Testes from all groups were removed and fixed in Bouin's solution and followed by dehydration in a descending series of ethyl alcohol, were cleared in xylene and embedded in paraffin. Paraffin sections of testes were cut at 5 μm on a rotary microtome, mounted on slides and stained with hematoxylin eosin (H&E) and examined under a light microscope by a blinded pathologist.Statistical Analysis:The data obtained in the present study were expressed as mean SD for quantitative variables and statistically analyzed by using SPSS program (version 18 for windows) (SPSS Inc. Chicago, IL, USA). One way Analysis of variance (ANOVA) is used to compare the results of all examined groups followed by LSD test to compare statistical differences between groups. Paired t. test was used to compare the initial and final body weights. P value <0.05 was considered statistically significant.
3. Results
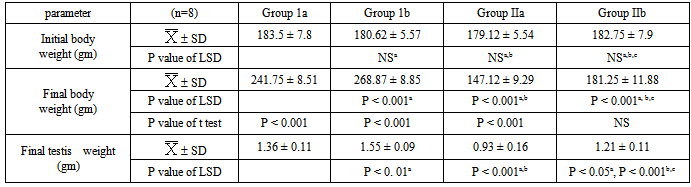
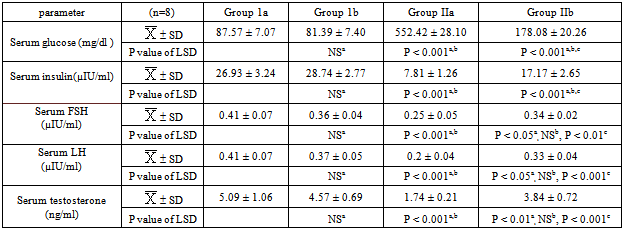
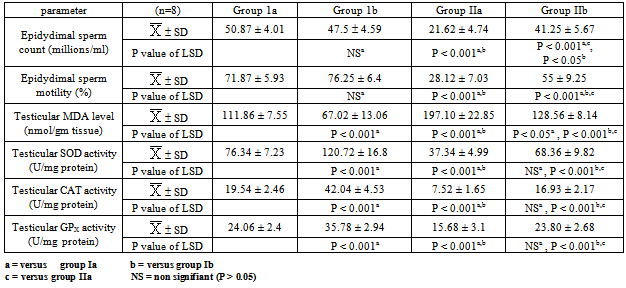
- The present study showed that STZ-induced diabetes significantly decreased the body weight, testes weight, serum insulin, FSH, LH & testosterone levels, epididymal sperm count & motility and testicular SOD, CAT & GPX activities and significantly increased serum glucose & testicular MDA levels (p<0.001) (tables 1, 2&3).
|
|
|
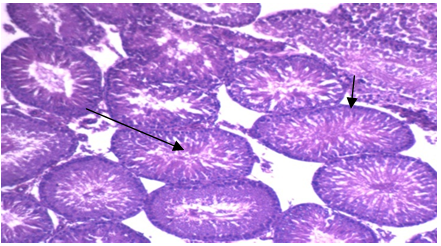
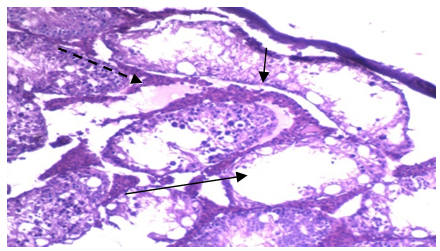
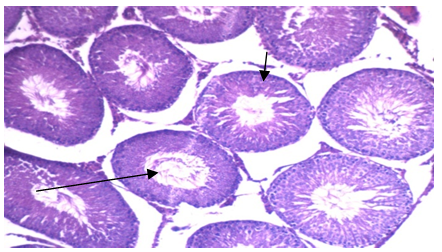
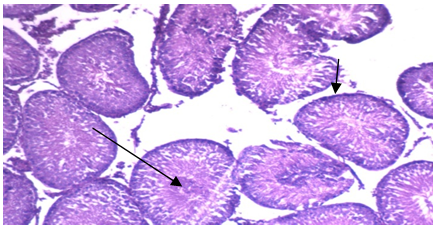 | Photo 2. Photomicrograph of of left rat testicular tissue isolated from group 1b showing: normal seminiferous tubules lined by normal thickness of the basement membrane (H&E - 400) |
4. Discussion
- Type 1 DM is associated with many complications including male infertility. Increased lipid peroxidation and imbalanced carbohydrate metabolism causes impairment of the steroidogenic function of the testis in addition to the cellular and tissue damage[47]. This may explain the possible etiologies for increasing infertility cases among diabetic males[48], and so the balance between oxidant and antioxidant species has been proposed to have an important role in preventing diabetic complications[12]. Ghrelin, a peptide hormone predominantly produced by the stomach and, it is the endogenous ligand for the growth hormone secretagogue receptor[49]. Its plasma levels are reduced in type 1 diabetes mellitus[50]. Moreover, ghrelin has been reported to exert antioxidant properties[51]. Therefore, we designed this study to explore the probable effects of this peptide in modulating the adverse effects of diabetes on testicular function.In present work, ghrelin administration significantly increased the body weight in both normal and diabetic groups. This is in accordance with the findings of other investigators who reported that ghrelin administration increased body weight of freely fed mature rats[52, 53]. This effect may be induced by a number of biological responses at the central neuroendocrine level, including stimulation of food intake and adiposity[54, 55, 56]. Both acylated and des-acylated ghrelin has been shown to cross the blood brain barrier (BBB) via either active transport or passive diffusion [57, 58]. The hypothalamic arcuate nucleus (ARC) is the main site of ghrelin’s activity in the central nervous system where ghrelin act via GHSR-1a to stimulate neurones containing the orexigenic factors neuropeptide Y and agouti related peptide and inhibit neurons producing the anorexigenic peptides pro-opiomelanocortin, cocaine and amphetamine related transcript[59]. Neurones in the arcuate nucleus also stimulate orexin-containing neurones in the lateral hypothalamic area to increase appetite[60]. The primary effect of ghrelin injections on meal patterns is a decrease of inter- meal intervals, thus increasing meal number without affecting meal size[61]. In addition to its orexigenic effects, ghrelin plays a role in the regulation of long-term energy homeostasis. Ghrelin increased the preference of fat diets & induced adipogenesis [62] and decreased adipocyte apopotosis[63], lipolysis[64], sympathetic nervous system activity, body temperature, and locomotor activity, therefore, ghrelin plays a role in all aspects of energy homeostasis, tending to promote weight gain[65]. In addition to the orexigenic and adipogenic effect of ghrelin, it may also recover the body weight of the diabetic group through the improvement in glucose homeostasis, which was demonstrated in our results.In the present work, i.p administration of STZ significantly elevated fasting serum glucose level with a significant concomitant reduction in serum insulin level as reported earlier by Shrilatha and Muralidhara,[66]. The mechanism of STZ induced DM is probably through the generation of ROS, leading to islet cell destruction[67]. In addition, it was found that while exogenous administration of ghrelin resulted in a significant recovery of serum glucose and insulin levels in STZ diabetic rats, it was associated with insignificant alterations of these parameters in the normal control rats. These results are in agreement with those of some investigators who reported that exogenous administration of ghrelin in low doses resulted in increase in insulin release which may be partly via the increase in intracellular Ca+2 concentration induced by activation of phospholipase C (PLC)[26,68,69].Also, our results are supported by the findings of Kerem et al.[70] who reported that in 90% pancreatectomized rats, acylated ghrelin (AG) administration strongly reduced glucose levels & increased insulin-producing β -cell number together with increased insulin secretion. On the contrary, they found that ghrelin receptor antagonist administration worsens glucose levels and β -cell mass that is in support of the involvement of GHSR1a or of endogenous ghrelin in islet cell survival and function.However, the results of the present work at variance with those of others who demonstrated that exogenous administration of ghrelin in high doses resulted in a significant increase in blood glucose level associated with a suppression of insulin release via opening of voltage sensitive K+ channels that repolarizes the plasma membrane and closes Ca2+ channels[25,71]. It is well established that chronic hyperglycemia in addition to production of ROS can directly promote an inflammatory state, where the increase in cytokines (IL-6, IL-18, IL-1 and TNF -α ) can lead to destruction of the pancreatic beta cells[72]. Interestingly, in the pancreas of STZ-diabetic rats, ghrelin was reported to promote cell proliferation and inhibit cytokines induced apoptotic events (specially by NO) in β-cell lines via Gs α protein-coupled receptor/ adenylyl cyclase/ cAMP/ protein kinase A pathway [73,74]. Thus, the correction of hypoinsulinemia and hyperglycemia encountered, in the present study, in STZ diabetic rats by exogenous administration of ghrelin could be attributed to the antioxidant protective effects of this peptide with promotion of β cell proliferation/regeneration (i.e. increased β cell mass) and increased insulin expression and secretion. The results of the present work also demonstrated a significant decrease in serum FSH, LH& testosterone levels in STZ-diabetic group in comparison to the normal control group. These results are in line with many investigators who attributed this to interference of diabetes with the hypothalamo-pituitary–testicular axis at multiple levels. They also showed that this impairment was induced by ROS & resulted in a significant decrease in expression of steroidogenic genes pathways[7,75,76,77]. However, our results are at variance with those of Idris et al.,[8] who reported that plasma testosterone and LH levels were unaffected in diabetic rats while FSH level was remarkably high and, observed that diabetes did not alter the functions of pituitary glands and Leydig cells but affected Sertoli cell functions owing to reduction in FSH receptors. Since Sertoli cells are involved in developing sperm cells and regulating internal environment of seminiferous tubules, reduction in their number decreases spermatogenic output[78].In fact GnRH-expressing neurons in the hypothalamus express insulin receptors that respond to insulin stimulation by showing an increase in the expression of the transcriptional factor (c-fos), rapidly followed by an increase in the expression of GnRH itself suggesting that hypothalamic GnRH neurons may be directly modulated by insulin[79]. Therefore, insulin deficiency in STZ-diabetic rats may reduce the strength of the GnRH impulse and consequently attenuate GnRH dependent pituitary feedforward signaling[6]. Herbison,[80] and Soudamani et al.[81] have also shown decreased availability of serum estradiol & catecholamine synthesis at the hypothalamic level in STZ-induced diabetic rats and this in turn leads to decreased GnRH pulse frequency and amplitude.Moreover, in vitro studies have shown that insulin may act directly on the anterior pituitary in the regulation of gonadotropin release[82], and alterations in the cellular composition of the anterior pituitary could also be involved in some of the diabetes-induced endocrine disruptions, as this gland undergoes increased ROS-induced apoptosis in poorly controlled STZ-diabetic rats[83]. The present study showed that the streptozotocin-induced diabetic rats had poor sperm quality. The Epididymal sperm count was significantly low with a significant decrease in sperm motility. These findings are consistent with some investigators[8,12,16], but varies with others who reported that diabetic men may have normal semen parameters or having a significant decrease in semen volumeand have a higher level of damage in sperm nuclear and mitochondrial DNA than control individuals[9,84]. Oxidative stress is one of the possible reasons for the changes in sperm quality observed in the present study in STZ-induced type I DM. The resulting mitochondrial dysfunction impairs spermatogenesis and decreases sperm motility[85]. Apart from that, sperm head membrane is also highly sensitive to oxidative stress due to rich polyunsaturated fatty acid content[86]. Current situation is worsened by the highly condensed DNA in sperm, where free radicals induce genotoxicity by initiating sperm DNA denaturation and fragmentation with limited DNA repair[87]. Another possible reason for low sperm count in STZ-diabetic group is low testicular glucose utilization by Sertoli cell which serve as energy source for sperm development[88] and decrease in sex hormones, another factor that can regulate spermatogenesis[8].The present findings showed that exogenous ghrelin administration resulted in a significant recovery of FSH, LH &testosterone levels together with a marked restoration in epididymal sperm count and motility towards the control levels in the STZ- induced diabetic group. These protective effects of this peptide may be due to its free radical scavenging properties, which were proved in the present study as it was found that while STZ-induced diabetes resulted in a significant elevation of the testicular MDA level accompanied with a significant reduction of testicular antioxidant enzymes CAT, GPX and SOD activities, exogenous administration of ghrelin to these diabetic animals resulted in a significant recovery of all these parameters.Since oxidative stress plays a key role in pathogenesis of DM induced male reproductive defects[16,89], it revealed that ghrelin possibly via its antioxidant properties protects the testicular germ cells against the adverse effects of DM, accelerates testicular regenerative processes in this condition and enhances the rate of total sperm motility & the percentage of sperm forward progressive movement that may be attributed to increased intracellular Ca+2 level after binding of ghrelin to its specific receptor[90,91].It is shown that STZ-induced diabetes significantly decreased the testicular weight in comparison to the normal control group. Moreover, histopathologically, testicular sections showed interstitial edema together with variable sized and shaped semineferous tubules lined by few layers of spermatogenic cells with wide lumina and marked thickening of the basement membrane in STZ-diabetic group. This is in accordance with the findings of Nah et al.[7]; Idris et al.[8] & Kyathanahalli et al.[92].Those adverse effects of diabetes on the testicular weight and architecture are likely due to low serum levels of both testosterone hormone which is the major regulator of normal growth of reproductive organs and FSH hormone. Since this hormone is essential to stimulate Sertoli cells to synthesize and secrete androgen-binding protein, which is important to bind testosterone that is required for terminal differentiation of spermatids[93]. Also, decrease in FSH level could also decrease the production of the stem cell factor (SCF). This factor is a Sertoli cell product that has been used in Leydig cell development and survival and is acting as a survival factor for the different cell types in the seminiferous epithelium such as spermatogonia in adult rats[94]. Furthermore, the physical alterations observed possibly resulted from testicular local reaction caused by the diabetic oxidative stress stimulating macrophages and other inflammatory cells to secrete cytokines as interleukin 1 β & tumour necrotic factor (TNF), and NO which affect both steroidogenesis and spermatogenesis[75,95] in addition to the metabolic disturbances due to insulin deficiency[96].In addition it was found that exogenous administration of ghrelin was associated with marked improvement in testicular histoarchitecture of diabetic rats together with significant recovery in the testicular weight. These findings could be attributed to the antioxidant properties of this peptide as was proved in the present study together with maintenance of nearly normal levels of LH, FSH, testosterone, glucose & insulin. One of outstanding features of the present study was that the observation that exogenous ghrelin administration in normal control rats produced no changes in testicular histoarchitecture together with insignificant changes in serum FSH, LH & testosterone levels and insignificant changes in eididymal sperm count & motility. However, it resulted in a significant increase in both testicular weight & testicular antioxidant enzymes activities but a significant decrease in testicular MDA level. These results are in agreement with those of many researchers who reported that chronic administration of ghrelin a) tended to increase testicular weight in fed animals without any significant change in plasma levels of sex hormones[97], b) increased the integrity of sperm membrane, however the sperm motility and concentration did not display any alterations[98], c) had antioxidant effects in rat testes[51], but in contrast to those of others who demonstrated that systemic administration of ghrelin reduced the GnRH pulse frequency in rats that is mediated by NPY and completely abolished by the NPY-Y5 receptor antagonist[99], and those concluded that, ghrelin can suppress both LH and FSH secretion in both male and female rats and humans[100, 101, 102]. It seems that the effects of ghrelin clearly depends on the nutritional state as it was observed that while ghrelin administration in fed rats did not induce detectable changes in plasma levels of gonadotropins and testosterone, in food-restricted animals, where endogenous ghrelin levels are known to be increased[103], it was found that chronic administration of ghrelin induced overt decreases in plasma LH and testosterone levels[97] and, such a state of suppression is produced by the massive hyper-ghrelinemia induced by the combined increase of endogenous ghrelin in calorie-restricted animals plus the exogenous administration of the peptide[104]. This observation is in agreement with previous reports showing a predominant inhibitory effect of high doses of ghrelin on LH and testosterone secretion in male rats[36,105]. Moreover, Antonyselvi et al.[53] emphasized that ghrelin suppresses different stages of spermatogenesis in normal albino rats in a dose dependent manner and that this suppression was more pronounced at a dose of 20μg/kg of body weight.The discrepancy between our results and those of others may be due to differences in the chosen dose, route of administration, duration of administration, species difference and / or nutritional status.In conclusion: While ghrelin produces no significant alteration in testicular function in normal rats, however it restored the testicular functions including steroidogenesis and spermatogenesis in STZ-diabetic rats to become nearly normal. The probable mechanisms of action of this peptide might be via 1) decreasing the lipid peroxidation as evidenced by reduction in MDA levels and by increasing the antioxidant enzymes SOD, CAT and GPx activities in the testes. 2) increasing LH, FSH & testosterone levels. 3) improving carbohydrate metabolism as evidenced by significant decrease in serum glucose which was accompanied by significant increase in serum insulin levels. All these mechanisms may be due to the antioxidant properties of this peptide at different levels such as hypothalamus, pituitary, testes and pancreas.Further studies are required to investigate the use of ghrelin as a potential protective target for the hypothalamo - pituitary-testicular axis dysfunction in diabetics.
ACKNOWLEDGMENTS
- To Prof. / Kamal EL-Kashish, pathology department - Medicine, Zagazig University for performing the histological study and to Prof. /Somiaa Hasan Biochemistry department-Medicine, Zagazig University for performing the laboratory tests.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML