-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2013; 2(6): 96-100
doi:10.5923/j.diabetes.20130206.02
Studies on Uric Acid Level in Hypertensive and Non-hypertensive Type 2 Diabetes Mellitus
Gospel Ajuru1, Miebi Wankasi2, Donatus Onwuli3
1Department of Anatomical Pathology, Faculty of Basic Medical Science, University of Port Harcourt, Choba, Rivers State, Nigeria
2Department of Medical Laboratory Science, Niger Delta University, Yenagoa, Bayelsa State, Nigeria
3Department of Medical Laboratory Science, Rivers State University of Science and Technology, Port Harcourt, Rivers State, Nigeria
Correspondence to: Gospel Ajuru, Department of Anatomical Pathology, Faculty of Basic Medical Science, University of Port Harcourt, Choba, Rivers State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
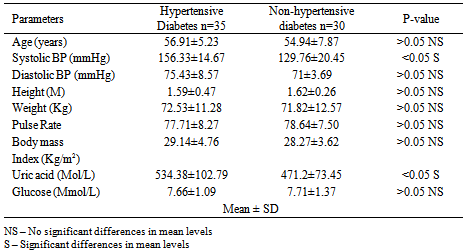
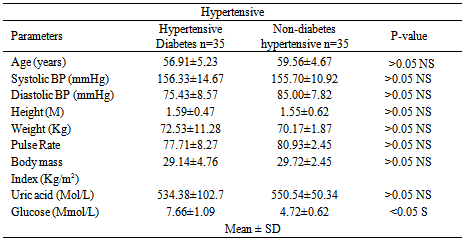
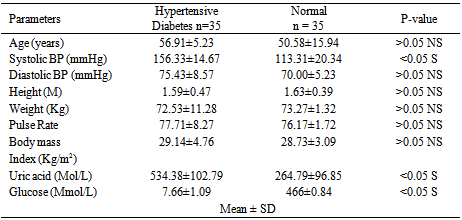
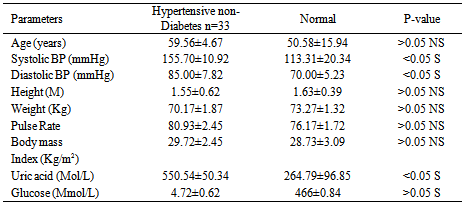
Studies on effect of serum uric acid in hypertensive and non-hypertensive type 2 diabetes was performed to determine whether these biochemical parameters such as age, systolic blood pressure, diastolic blood pressure, height, weight, pulse rate, body mass index, and glucose were associated with these disease conditions. A total of one hundred and thirty three (133) individuals were used for this study. Thirty five (35) individuals were established hypertensive diabetes and thirty (30) were established non-hypertensive diabetes. The established hypertensive non diabetes was thirty three (33) while thirty five (35) were normal healthy individuals (non-hypertensive and non-diabetes). The results showed that the mean level of uric acid was significantly higher (p< 0.05) in established hypertensive diabetes compared to non- hypertensive diabetes. It was observed that uric acid as well as systolic and diastolic blood pressure was significantly higher (p> 0.05) in hypertensive non diabetes individuals compared with normal healthy individuals studied. However, the glucose and pulse rate mean levels showed no significant difference (p< 0.05) between hypertensive non-diabetes and normal healthy individuals. The significant biochemical effects observed are important to the diagnosis and treatment of these chronic metabolic disorders. Also, hypertension and diabetes are independent pathological conditions
Keywords: Hypertensive type 2 diabetes, Serum uric acid, Non-hypertensive type 2 diabetes, Systolic blood pressure, Diastolic blood pressure, Glucose, Pulse rate
Cite this paper: Gospel Ajuru, Miebi Wankasi, Donatus Onwuli, Studies on Uric Acid Level in Hypertensive and Non-hypertensive Type 2 Diabetes Mellitus, International Journal of Diabetes Research, Vol. 2 No. 6, 2013, pp. 96-100. doi: 10.5923/j.diabetes.20130206.02.
Article Outline
1. Introduction
- Cardiovascular diseases remain the major cause of morbidity and death in developing countries and accounts for approximately 40% of all deaths in Africa[1] Smoking and reduction in blood pressure have since shown to be effective strategies in the prevention of cardiovascular disease. However, the major classic risk factors and non-modifiable risk factors as age, sex, and family history cannot fully explain why some persons develop myocardial infarction, stroke, and other cardiovascular disease while others do not[2]. However, hypertension, cancer, diabetes, and cardiovascular disease account for nearly two of every three deaths in the United States in 2001. These diseases shorten life expectancy, undermine health, and cause enormous suffering, disability, and economic costs. Although most of this disease burden could be avoided if there were systematic application of what is known about preventing the onset and progression of these conditions. Hypertension is defined as rise in blood pressure. Normal blood pressure at rest is between 95/55 – 140/90 normally read as systolic blood pressure (SBP) and diastolic blood pressure (DBP) respectively; where systolic blood pressure is when the heart is at rest and relaxed while diastolic blood pressure is when the heart is contracted. Hypertension is strongly associated with hyperuricemia. It has been reported that serum uric acid are elevated in hypertension and are present in 25% of untreated hypertensive subjects taking diuretics and greater than 75% of patient with malignant hypertension. The mechanisms involved with the association of hyperuricemia and hypertension include, decreased renal blood flow (decreased GFR) and stimulate urate reabsorption resulting in local tissue ischemia[4]. Uric acid is the major product of the catabolism of the purine nucleosides, (adenosine and quanosine). It is obtained from catabolism of dietary nucleic acid is converted to uric acid[5]. The daily synthetic rate of uric acid is approximately 400mg; 300mg contribute to the dietary sources. 1200mg of men consume purine-free diets are estimated, while in women, the estimated value is 600mg. However, individuals with gouty arthritis and tissue deposition of urate may have urate pools of between 18,000 to 30,000mg[6]. Over secretion of uric acid may result from purine precursors synthesis. Approximately 75% of the uric acid excreted by human is secreted in the urine; while most of the remainder is secreted in the gastrointestinal tract (GIT), where allantoin and bacteria compound enzymes are degraded. Renal handling of uric acid is complex and involves four sequential steps: (1) Glomerular filtration, (2) Re-absorption of about 98% to 100% in the proximal convoluted tubule, (3) Secretion into the lumen of the distal proximal tubule by an ATPase – dependent mechanism that contributes to overall clearance[7], (4) Re-absorption in the distal tubule. An epidemiological studies between elevated serum uric acid and an increased cardiovascular risk has been reported[8]. It has been reported that serum uric acid concentrations are higher in patient with established coronary heart disease compared with healthy controls[9]. However, hyperuricemia has also been associated with elevated serum triglyceride and cholesterol concentration, and body mass index[8]. Symptomic hyperuricemia has been predicted for future development of hypertension, irrespective of renal function[2]. Among patients with elevated hypertension, serum uric acid concentration has been associated with a significantly increased cardiovascular risk[1]. Reports show that elevated serum uric acid may act as a marker of local or systemic tissue ischemia and provides one possible explanation for a non-causal associated link between hyperuricemia and cardiovascular disease[10]. It was therefore, concluded that a link between elevated serum uric acid concentration and cardiovascular disease may arise through its non-causal relationship with insulin resistance syndroms[11]. Type 2 diabetes is of particular concern because it is so common and usually occurs in persons of advancing age, when multiple other risk factors co-exist. There is a growing consensus that most patients with diabetes mellitus, especially those with type 2 diabetes, belong to a category of high short-term risk when the risk factors of diabetic patients are summed. Their risk often approaches that of patients with (established) coronary heart disease[12]. The prevalence of type 2 diabetes, the most common form of diabetes, is steadily increasing. In addition, in most ethnic groups, the rates for type 2 diabetes are higher in women than in men[13]. The majority of these studies suggest that type 2 diabetes increases the risk of coronary heart disease to a greater extent in women than men.
2. Materials and Methods
- The materials used for the study were reagents (Kits) for the determination of uric acid and blood glucose. A total of one hundred and thirty-three (133) individuals were used. Thirty five (35) individuals are known to be established hypertensive diabetes while thirty (30) are known to be established non-hypertensive diabetes. Thirty three (33) individuals are established hypertensive non-diabetes and thirty five (35) are normal healthy individuals who are non-hypertensive and non-diabetic. The subjects were patients recruited from Madonna University Teaching Hospital, Elele, Rivers State, and Braithwaite Memorial Hospital, Port Harcourt, Rivers State, Nigeria. The normal healthy individuals were volunteered staff of Madonna University, Elele and Rivers State College of Health Science and Technology, Port Harcourt, Rivers State, Nigeria. After an overnight fasting blood samples (5ml) was obtained from all subjects, blood samples were processed within two to three hours. Blood were collected in a main specimen bottle without anticoagulant; let to clot, span at 150rpm for 10 minutes and serum was separated and stored at 4oc until assayed. Blood for glucose determination/estimation was collected into specimen bottle containing fluoride oxalate as anticoagulant.
2.1. Physical Measurements
- The physical parameters such as blood pressure, height, and weight were measured from the individuals prior to blood collections. Height was measured without shoes to the nearest cm using a ruler attached to the wall. Weight was measured to the nearest 0.1kg on an electronic scale weighing balance. The body mass index was calculated as:
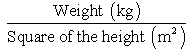 | (1) |
2.2. Principles and Procedures of Methods
2.2.1. Determination of Uric Acid
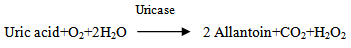 2H2O2 + 3,5-Dichloro-2-hydrocybenzenesultonic acid + 4-aminophenazone
2H2O2 + 3,5-Dichloro-2-hydrocybenzenesultonic acid + 4-aminophenazone  N-(4-antipyryl)-3-chloro-5- sulfonate P-benzo-quinonimineUric acid is converted by uricase to allantion and hydrogen peroxides. In the present of peroxides, hydrogen peroxide reacts oxidatively with 3,5-dichloro-2- hydroxybenzensulfonic acid and 4-aminophenazone to form a red dye. Potassium ferricyanide is included in the reagent in order to oxidize ascorbate in the serum that competitively interferes with the reaction. The absorbance read at 280nm.
N-(4-antipyryl)-3-chloro-5- sulfonate P-benzo-quinonimineUric acid is converted by uricase to allantion and hydrogen peroxides. In the present of peroxides, hydrogen peroxide reacts oxidatively with 3,5-dichloro-2- hydroxybenzensulfonic acid and 4-aminophenazone to form a red dye. Potassium ferricyanide is included in the reagent in order to oxidize ascorbate in the serum that competitively interferes with the reaction. The absorbance read at 280nm.2.2.2. Procedure
- Into the labeled test tubes containing 20ml of standard and test, 1ml of reagent was added, including the blank test tube. These were mixed and incubated for 5 minutes at 37℃. The absorbance of test and standard was measured against blank. Uric acid concentration was obtained by dividing absorbance of standard and multiplying by concentration of standard. Result was expressed in µmol/L.Calculation: The concentration of uric acid was calculated using the formula:
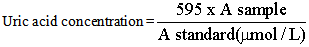 Where:A sample = Absorbance of sampleA standard= Absorbance of standard
Where:A sample = Absorbance of sampleA standard= Absorbance of standard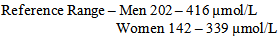
2.3. Determination of Blood Glucose
2.3.1. Principles
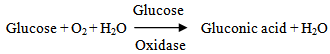 The blood glucose was determined according to the enzymatic calorimetric method. The enzyme glucose oxidase catalyzes the oxidation of glucose to produce hydrogen peroxide and gluconic acid. In the presence of hydrogen peroxidase, the hydrogen peroxide is broken down and the oxygen released reacts with 4-aminophenazone and phenol to quinonamine. The color intensity determined by absorbance is proportional to the glucose concentration.
The blood glucose was determined according to the enzymatic calorimetric method. The enzyme glucose oxidase catalyzes the oxidation of glucose to produce hydrogen peroxide and gluconic acid. In the presence of hydrogen peroxidase, the hydrogen peroxide is broken down and the oxygen released reacts with 4-aminophenazone and phenol to quinonamine. The color intensity determined by absorbance is proportional to the glucose concentration.2.3.2. Procedure
- Serum and reagents were mixed in suitable labeled centrifuge tubes, and centrifuged at 2500rpm for 5 minutes. 1.0ml supernatant was transferred to another test-tube and 3.0ml colour reagent was added. For the blank, 1.0ml protein precipitant and 3.0ml colour was used. It was well mixed and incubated in tubes at 37oC for 10 minutes, shaken occasionally to ensure adequate aeration. The tubes were removed from the water bath, cooled and measurement of the absorbance against the blank at 515nm (qreen Filter) in 1cm cell was taken.
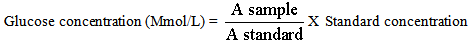 | (2) |
3. Results
|
|
|
|
4. Discussion
- Diabetes mellitus is a common chronic metabolic disorder of glucose and fat[14]. Its prevalence is increasing worldwide. However, some diabetes are also afflicted by conditions such as hypertension. The uric acid levels in established hypertensive diabetes compared to non-hypertensive diabetes indicates that hypertensive diabetes have higher concentration of uric acid. This could be attributed to increased uric acid retention induced by certain diuretic containing agents used to lower blood pressure by hypertensive diabetes. A previous study had also demonstrated that increased use of certain diuretic drugs reduce the excretion of uric acid, which may lead to hyperuricemia due to uric acid retention[15]. Those of major interest include Thiazides, Furosemide, and Ethancrynic acid diuretics. The biochemical parameters estimated were observed to show no significant differences between hypertensive diabetes and non-diabetes hypertensive studied, except for serum glucose concentration that was found to be higher in hypertensive diabetes. However, the elevated blood glucose (hyperglycemia) observed is equated with diabetes. This finding also indicates that hypertension and diabetes are independent pathophysiological conditions. This is consistent with previous reports which attributes diabetes mellitus to be due to insulin malfunction and the result of insufficient action of insulin in an increase in blood glucose while hypertension is caused by several factors, for which in most cases are unknown.
5. Conclusions
- The significant biochemical effects observed are important in the diagnosis and treatment of these chronic metabolic disorders. And also, these disease conditions, hypertension and diabetes mellitus are independent pathophysiological conditions even though there seems to be an association between hypertension, diabetes mellitus and insulin resistance, However, it is particularly challenging since many of the agent used to lower blood pressure can affect glucose metabolism adversely.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML