-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2013; 2(5): 84-89
doi:10.5923/j.diabetes.20130205.01
Comparative Effects of Aqueous Leaf Extract of Viscum album (Mistletoe) and Aloe vera gel in the Management of Streptozotocin - Induced Diabetes Mellitus
V. U. Nna 1, V. O. Oka 1, E. O. Aluko 2, O. T. Helen 2
1Department of Physiology, Faculty of Basic Medical Sciences, University of Calabar, Calabar, Cross River State, Nigeria
2Department of Physiology, Faculty of Basic Medical Sciences, University of Uyo, Uyo, Akwa Ibom State, Nigeria
Correspondence to: V. U. Nna , Department of Physiology, Faculty of Basic Medical Sciences, University of Calabar, Calabar, Cross River State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study was aimed at comparing the efficacy of leaf extract of mistletoe with that of Aloe vera gel in the management of type 1 diabetes mellitus (T1DM). Thirty six albino wistar rats weighing 180 - 220 g were randomly divided into 6 groups of 6 rats each, thus; Group 1 - control; group 2 - diabetic untreated group; group 3 - diabetic group, treated with 0.4 ml/100g Aloe vera gel; group 4 - diabetic group, treated with 150 mg/kg mistletoe leaf extract; group 5 - control animals, treated with 0.4 ml/100g Aloe vera gel; group 6 - control animals, treated with 150 mg/kg mistletoe leaf extract. Administration of extracts was done per oral route and lasted for 21 days. Fasting blood glucose, water intake, food intake and body weight were measured during the study. Mistletoe treated diabetic group had a significantly (P<0.001) lower fasting blood glucose (FBG) level compared to diabetic untreated group. Aloe vera treated diabetic group had a significantly (P<0.001) lower FBG level compared to mistletoe treated diabetic group. Both extracts significantly (P<0.001) reduced mean daily water and food intake in the diabetic rats. Aloe vera treated diabetic group had a significantly (P<0.05) lower mean daily food and water intake compared to mistletoe treated diabetic group. Both extracts significantly (P<0.001) increased body weight in the diabetic rats. Aloe vera treated diabetic group had a significantly (P<0.001) higher body weight compared to mistletoe treated diabetic group. The Aloe vera treated control group had a significantly (P<0.001) higher body weight compared to mistletoe treated control group. Unlike Aloe vera gel which increased the body weight of diabetic and normal animals significantly, mistletoe leaf extract was found to increase the body weight of diabetic animals minimally and reduced the body weight of normal animals significantly (P<0.05) compared to control. On the basis of the results obtained from this study, we therefore conclude that Aloe vera gel proved to be more beneficial in the management of type 1 DM compared to mistletoe leaf extract.
Keywords: Aloe vera, Blood glucose, Body weight, Hyperglycemia, Mistletoe, Viscum album
Cite this paper: V. U. Nna , V. O. Oka , E. O. Aluko , O. T. Helen , Comparative Effects of Aqueous Leaf Extract of Viscum album (Mistletoe) and Aloe vera gel in the Management of Streptozotocin - Induced Diabetes Mellitus, International Journal of Diabetes Research, Vol. 2 No. 5, 2013, pp. 84-89. doi: 10.5923/j.diabetes.20130205.01.
Article Outline
1. Introduction
- Diabetes mellitus (DM) has been classified as a metabolic disorder characterized by hyperglycemia, that has reached epidemic proportions in recent time[1]. Several drugs and plant materials are presently in use to reverse hyperglycemia in diabetics. Some of these materials negatively influence fluid intake, food intake and body weight, thus, the search for a new class of compounds has become necessary to overcome these side effects[2]. Considering the fact that DM is a metabolic disorder, it is important to evaluate and understand the impact of the disease on the metabolic needs of the body.Aloe vera is a succulent perennial plant with over 350 species. It belongs to family Liliaceae, native to North Africa and cultivated in warm climatic areas[3]. Aloe vera has been promoted as a remedy to a large variety of conditions. Of the over 350 species, Aloe vera barbadensis is the species that is most medicinally effective[4]. Aloe can be utilized therapeutically, in three forms – aloe latex, aloe gel and aloe whole leaf extract. Aloe vera gel is well known for its healing potentials. The latex of Aloe vera contains the anthraquinone glycosides aloin A and B, which are potent laxatives[5,6]. Aloe vera has been reported to be beneficial in the management of ailments in the following systems - cardiovascular system, endocrine system, respiratory system , blood and immune system[6-10].Viscum album (Mistletoe) is an obligate parasite, belonging to family Loranthaceae. It is widely distributed across Europe, Australia, Asia and Africa. Mistletoe obtains part of its food from its host plant. It depends on its host for minerals and water, but obtains carbohydrate by the process of photosynthesis[11]. Extracts of mistletoe leaf have been found useful in the treatment of liver disorders, epilepsy, cholera, cancer, hypertension and diabetes, most of which are yet inconclusive[12-15].The incidence of diabetes mellitus has been on the increase in recent times. With the ease of accessibility of plant materials, especially in the rural population, who depend almost entirely on plant materials for medicinal purposes, it became necessary to investigate the antidiabetic effects of mistletoe leaf extract and Aloe vera gel which are widely in use in rural areas, with a view to better inform the populace about the effect of these extracts on blood glucose levels, fluid intake, food intake and body weight.
2. Material and Methods
2.1. Plant Material and Preparation of Plant Extracts
- Fresh mistletoe leaves were obtained from a citrus host plant in Calabar South local government area of Cross River State, Nigeria. Fresh mature (up to 2 years old) Aloe vera leaves with length between 50 - 60 cm were obtained from University of Calabar botanical garden. Both plants were identified by the chief herbarium officer of Botany department in University of Calabar, Cross River State, Nigeria. The mistletoe leaves were rinsed in clean water to remove debris. They were sun dried and subsequently transferred into the AstellHearson oven and dried at 40 - 45℃. The dried leaves were ground to powder using an electric blender. 1000 g of the dry sample was mixed with 5000 ml distilled water and allowed to stand for 24 hours. The mixture was then filtered with Whatman’s filter paper (size 1). The filtrate was dried at 45℃ using AstellHearson oven. The pasty filtrate obtained after drying was weighed using a mettler P163 electronic weighing balance. The stock solution of the extract was prepared by dissolving 15 g of extract in 10 ml of distil water to give a concentration of 1500 mg/ml. The stock solution was appropriately labeled and refrigerated at 4℃ until required for use. This preparation was done weekly to avoid modification of extract due to prolonged storage.The Aloe vera leaves were rinsed in clean water to remove debris and sand. A knife was then used to slice the leaf longitudinally to expose the crude Aloe vera gel. The gel was scraped gently into an electric blender to shatter the block. Care was taken not to scrape too deep into the Aloe leaf to avoid the Aloe vera latex which is completely different from the Aloe vera gel. The gel was administered to the animals in that form without modification or any additives. This procedure was repeated daily to avoid alterations of the crude gel due to storage. The median lethal dose (LD50) of the plant extracts were determined by method of Lorke[16]. Mistletoe leaf extract and Aloe vera gel were not found harmful at the highest tested dose of 500 mg/kg and 32 ml/kg respectively.
2.2. Animal Preparation and Protocol
- Thirty six (36) albino wistar rats weighing 180 - 220 g were randomly assigned one of six groups, such that each group contained six (6) animals. The groups were labeled as follows: Group 1 - control; group 2 - diabetic untreated group (DM); group 3 - diabetic group, treated with Aloe vera gel (DM+A); group 4 - diabetic group, treated with mistletoe leaf extract (DM+M); group 5 - control group, treated with Aloe vera gel (C+A) and group 6 - control group, treated with mistletoe leaf extract (C+M).
2.3. Induction of Diabetes
- Type 1 diabetes mellitus was induced by a single intraperitoneal administration of streptozotocin (STZ) at a dose of 65 mg/kg after 18 hours fast. Diabetes was confirmed 48 hours after STZ injection by the symptoms of polyuria and glucosuria. This state was confirmed using uristic test strip (Bayer Health Care LLC, USA). Also, blood glucose level was measured 48 hours after induction of diabetes using a Finetest glucose meter (INFOMED IMPEX, INDIA). Animals with fasting blood glucose level >200 mg/dl were considered diabetic and selected for this study.
2.4. Extract Administration
- Extract administration commenced 5 days after successful diabetes induction. Mistletoe leaf extract and Aloe vera gel were orally administered to DM+M; C+M and DM+A; C+A groups at a dose of 150 mg/kg and 0.4 ml/100g respectively, daily, for 21 days. Administration was facilitated by the use of a syringe and orogastric tube. All experiments regarding the animals and their care were in line with ethical standards laid down in the 1964 Declaration of Helsinki.
2.5. Determination of Fasting Blood Glucose Level
- Fasting blood glucose level of each animal was measured by the use of the Finetest glucose meter (INFOMED IMPEX, INDIA). Blood used for this purpose was obtained by pricking the distal end of the tail and placing the drop of blood on the glucose meter test strip. Blood glucose level before and 48 hours after STZ administration was determined and recorded in all the groups. Blood glucose level was also measured at the expiration of 21 days.
2.6. Measurement of Water Intake
- Daily water intake was obtained using a measuring cylinder and a calibrated conical flask. The daily water intake was obtained by subtracting the volume of water remaining at the end of 24 hours of feeding from the initial amount in the cylinder at the start of each day’s feeding[17].
2.7. Measurement of Food Intake
- Daily food intake was obtained by giving a measured quantity of rat feed; palletized Guinea feed, (Guinea Feed Ltd, Nigeria) each day and weighing the quantity remaining, same time the next day. The difference in quantity was recorded as the food intake. The recording was done at the same time daily to ensure consistency and accuracy[17].
2.8. Measurement of Body Weight
- The body weight of the animals was determined by using a weighing balance. The initial body weight of each animal was recorded before commencement of treatment with the extracts under evaluation. The rats were subsequently weighed every two days until the end of the study. The body weight was recorded and the body weight change was statistically analyzed.
2.9. Statistical Analysis
- All results are presented as mean ± standard error of mean. One way analysis of variance (ANOVA) was used to analyze results, followed by the least significant difference (LSD) procedure for significant F values. P = 0.05 was considered significant. Computer software SPSS and Excel Analyzer were used for the analysis.
3. Results
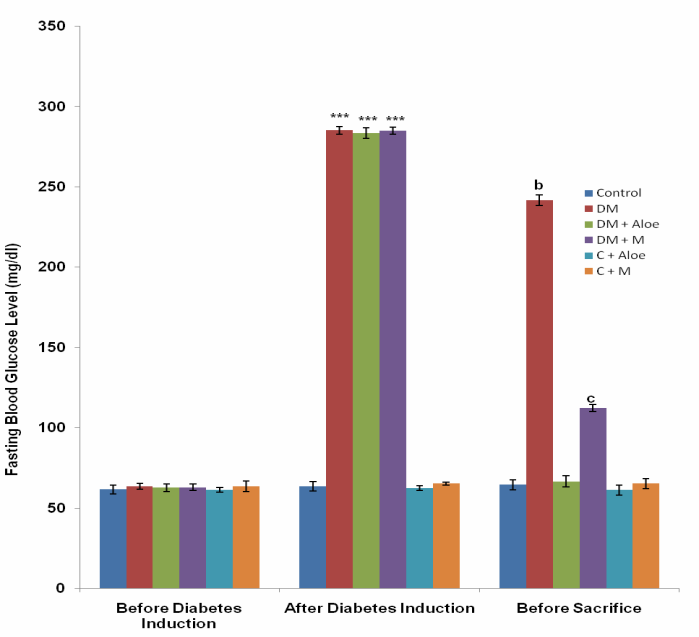
3.1. Fasting Blood Glucose Level in the Different Experimental Groups
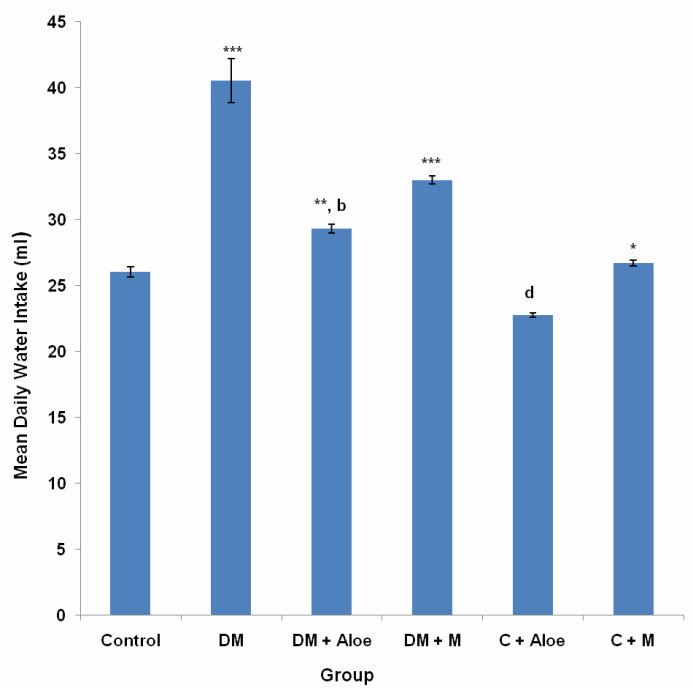
3.2. Mean Daily Water Intake
- Mean daily water intake in the control, DM, DM+A, DM+M, C+A and C+M group was 26.05±0.41, 40.56 ± 1.67, 29.32 ± 0.33, 33.03 ± 0.30, 22.78 ± 0.17 and 26.73 ± 0.23 ml respectively. Mean daily water intake was significantly (P<0.001) higher in DM and DM+M groups compared to control, DM+A, C+A and C+M groups. DM+A group had a significantly (P<0.05) lower mean daily water intake compared to DM+M. Mean daily water intake was significantly (P<0.05) lower in C+A group compared to control and C+M group (Fig. 2).
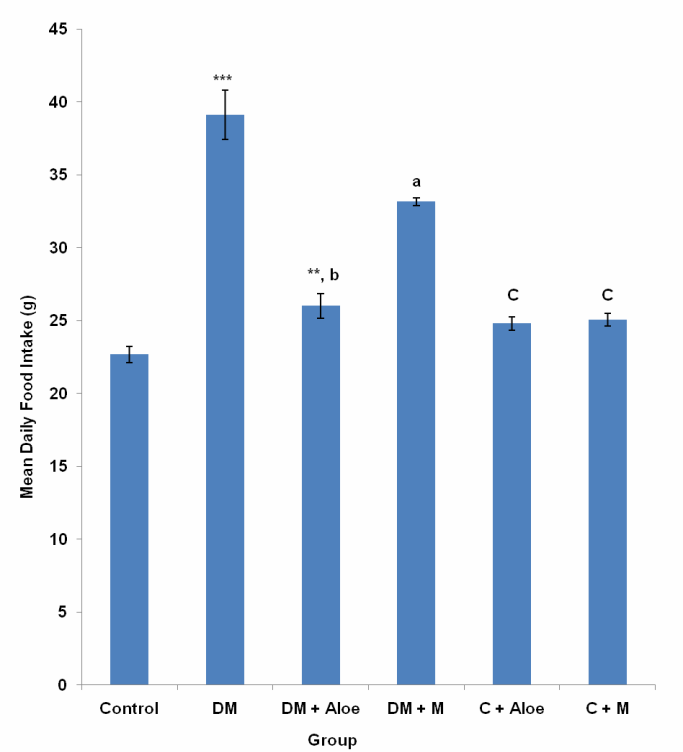
3.3. Mean Daily Food Intake
- Mean daily food intake in the control, DM, DM+A, DM+M, C+A and C+M group was 22.66 ± 0.56, 39.10±1.68, 25.99 ± 0.84, 33.15 ± 0.29, 24.79 ± 0.47 and 25.04 ± 0.45 g respectively. Mean daily food intake was significantly (P<0.001) higher in DM and DM+M groups compared to control, DM+A, C+A and C+M groups. Mean daily food intake in DM+A group was significantly (P<0.05) higher compared to control, and significantly (P<0.001) lower compared to DM+M (Fig. 3).
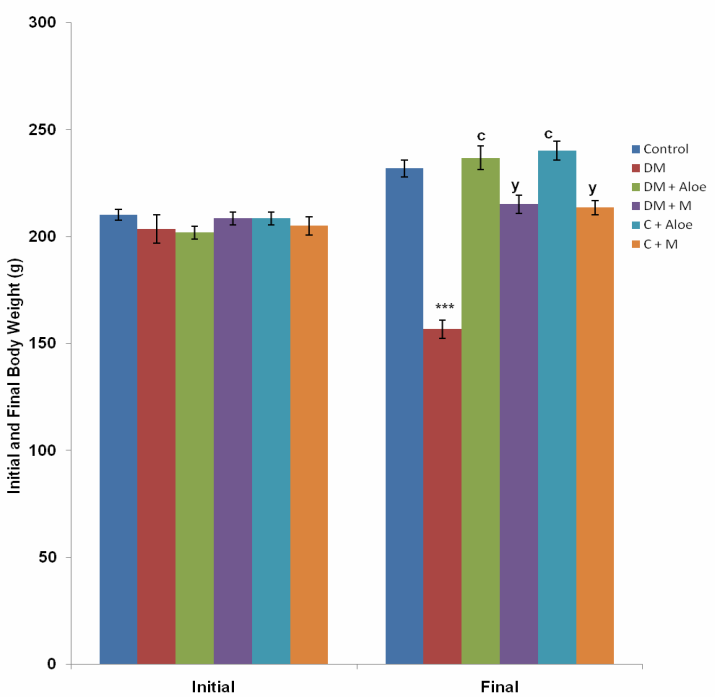
3.4. Body Weight
3.5. Body Weight Change
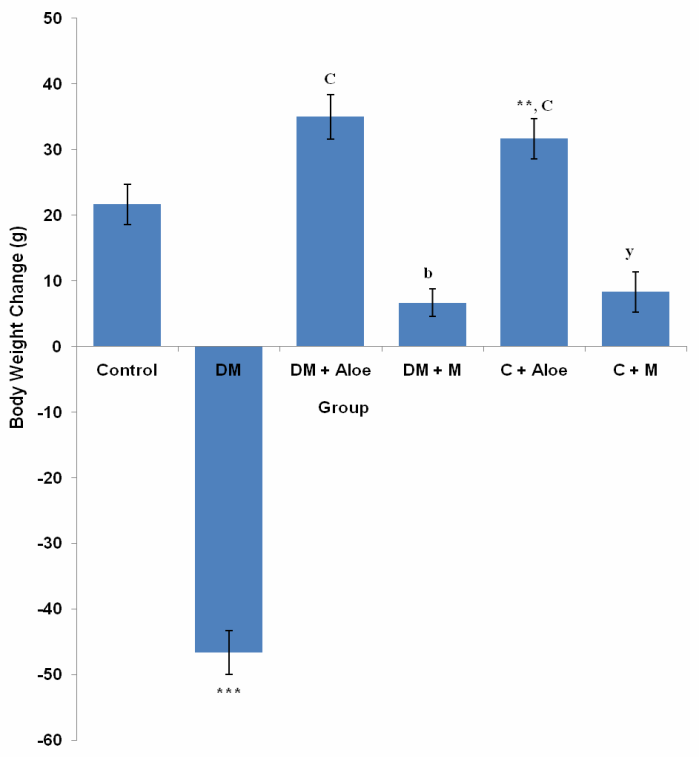
- Mean body weight change in the control, DM, DM+A, DM+M, C+A and C+M group was 21.7 ± 3.1, -46.7 ± 3.3, 35.0 ± 3.4, 6.67 ± 2.1, 31.7 ± 3.1 and 8.33 ± 3.1g respectively. DM group had a significantly (P<0.001) lower body weight change compared to control, DM+A, DM+M, C+A and C+M group. Body weight change was significantly (P<0.001) higher in DM+A and C+A group compared to DM+M and C+M group respectively. C+A group had a significantly (P<0.05) higher body weight change compared to control. C+M group had a significantly (P<0.05) lower body weight change compared to control (Fig. 5).
4. Discussion
- Diabetes mellitus (DM) could result from destruction of the pancreatic better cells or from deficient uptake of glucose by the body cells or both. Determination of fasting blood glucose (FBG) levels 48 hours after STZ administration confirmed diabetes mellitus in the groups administered (DM, DM+A and DM+M). STZ induced type 1 DM by destroying the pancreatic beta cells, thus limiting insulin production and secretion.Although mistletoe leaf extract significantly (P<0.001) reduced FBG levels of diabetic rats, Aloe vera gel decreased FBG level most significantly (P<0.001) compared to mistletoe leaf extract, (Fig. 1). This is in line with the work of Ekhaise et al[18] and Nwanjo[19], which showed that although mistletoe leaf extract lowered FBG level in the diabetic treated group, it did not completely reverse FBG levels to the level of that of the control animals. Both extracts did not reduce FBG levels in normal animals. This development is suggestive of a possible negative feedback mechanism that ensures FBG levels does not become too low as to cause hypoglycemia which is more disastrous. This is in line with reports that Aloe vera gel reduced blood glucose levels in diabetic rats[17]. In contrast to Obatomi et al[20] and Swanston-Flatt et al[21], Mistletoe leaf extract did not reduce fasting blood glucose levels of normal animals in our study (Fig. 1).Polydipsia was significantly (P<0.001) reduced by both extracts in our study. However, mean daily water intake was significantly (P<0.05) lower in DM+A group compared to DM+M group. This shows that Aloe vera gel was better off in reversing polydipsia as a symptom of DM compared to mistletoe leaf extract. C+A group had a significantly (P<0.05) reduced water intake compared to control. Suggesting a possible suppression of the thirst centers. Mistletoe leaf extract did not affect water intake in normal animals, (Fig. 2).Polyphagia was significantly (P<0.001) reduced by both extracts. However, Aloe vera gel significantly (P<0.001) reduced food intake in DM compared to mistletoe leaf extract, (Fig. 3). Body weight was significantly (P<0.001) reduced in DM group compared to control. This is as a result of STZ's activity on the pancreatic beta cells. Since the body cells will persistently require glucose, fats and proteins are mobilized to produce glucose and hence energy. This accounts for the depletion of energy stores and weight loss in type 1 DM. Aloe vera gel significantly (P<0.001) increased body weight of animals in DM+A group. Additionally, Aloe vera gel increased body weight of normal, non diabetic animals in C+A group, (Fig. 4 and 5). This development may be attributed to the presence of some enzymes in Aloe vera gel (alkaline phosphatase, amylase, carboxypeptidase, catalase, cellulase, lipase and peroxidase), some of which are involved in the breakdown of food sugars and dietary fats[17]. Lesions in the lateral hypothalamus (hunger center) leads to anorexia and weight loss while lesions in the satiety center leads to overeating and obesity, thus, suggesting a possible relationship between food intake and weight gain. On like Aloe vera gel, mistletoe leaf extract did not significantly raise the body weight of diabetic animals to the levels of that of control (Fig. 4 and 5). Indicating that mistletoe leaf extract did not completely reverse T1DM as evident in the results of FBG, food and water intake (Fig. 1, 2 and 3). Furthermore, mistletoe leaf extract significantly reduced the body weight of normal animals (C+M group), without influencing their food intake (Fig. 3, 4 and 5). This effect can be linked to the presence of tannins in mistletoe leaf extract. Tannins have been proven to inhibit adipogenesis[22]. This is suggestive of a possible beneficial effect of mistletoe leaf extract in managing obesity[23] rather than type 1 DM.
5. Conclusions
- On the basis of results obtained from this study, Aloe vera gel proved to be more beneficial in the management of type 1 DM compared to mistletoe leaf extract. Mistletoe leaf extract despite increasing food intake in diabetic treated group, did not increase their body weight to levels observed in the control group. This is probably due to the fact that it did not completely reverse T1DM as evident from our results. It also reduced the body weight of normal animals without influencing their food intake. This development is suggestive of a beneficial effect of mistletoe leaf extract on weight management. However, further research into this finding is necessary.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML