-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2013; 2(4): 76-80
doi:10.5923/j.diabetes.20130204.03
Cytogenetic and Immunogenic Study of Type -2 Diabetes Mellitus Patients
Azhar M. Hleem1, Salah M. Haleem2
1Head of Environmental Technology Department/ Environmental Research Center/University of Technology, Baghdad, Iraq
2Ministry of health /Al-Kindy hospital, Baghdad, Iraq
Correspondence to: Azhar M. Hleem, Head of Environmental Technology Department/ Environmental Research Center/University of Technology, Baghdad, Iraq.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
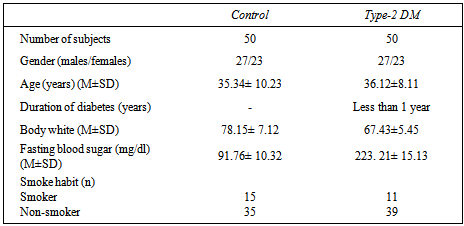
The current study which lasted from January to March (2010), the samples were collected from 50 untreated type-2 DM patients in different ages 18-65 years and sex-matched healthy control subjects were collected from specialized center for endocrinology and diabetes. The somatic cell chromosomal instability was assessed in peripheral blood lymphocytes. Chromosomal analyses (Mitotic index, Blastogenic index, Chromosomal aberration) were measured as well as phgocytosis index and percent. The study results showed that chromosomal state effected significantly in the DM patients by reducing both of mitotic index and blastogenic index while the chromosomal aberrations increased gradually according to the glucose level in blood and age. The immunogenic state effected by decreasing both phagocytosis percent and phagocytosis index significantly in the DM patients according to age.
Keywords: Keyword Cytogenetic, Mitotic Index, Phagocytosis, Diabetic Mellitus II, DNA Damage, Chromosomal Aberrations
Cite this paper: Azhar M. Hleem, Salah M. Haleem, Cytogenetic and Immunogenic Study of Type -2 Diabetes Mellitus Patients, International Journal of Diabetes Research, Vol. 2 No. 4, 2013, pp. 76-80. doi: 10.5923/j.diabetes.20130204.03.
Article Outline
1. Introduction
- Diabetes mellitus is a group of metabolic diseases characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both[1] Diabetes mellitus represent a serious health problem affecting millions of individuals worldwide, by the year 2025; the World Health Organization (WHO) predicts that 300 million people will have diabetes mellitus[2]. Oxidative stress is thought to play a significant role in the aetiopathology of type -2 diabetes mellitus[3, 4]. Previous studies have clearly shown that ROS, including O2−, OH−, and H2O2, are highly reactive and capable of damaging cellular macromolecules, including proteins, lipids and DNA[4, 5] Moreover, individuals with diabetes mellitus have reduced antioxidant defense capacity[5]. It has been demonstrated that diabetes mellitus is associated with elevated level of oxidative DNA damage and with the increased susceptibility to mutagens and the decreased efficacy of DNA repair[6, 7]. This can contribute to the chromosomal instability in diabetics. Several in vitro assays have been developed for measurement of genotoxicity, blastogenic index, mitotic index, chromosomal aberration assay in lymphocytes are being used extensively to evaluate the presence and extent of chromosomal damage in human populations. There are limited reports on the association between diabetes and the occurrence of genotoxic effects[8] reported that there is a significant increasing in sister chromated exchange and micronuclei in type -1 diabetes mellitus. The mitotic index assay good indicator to study the chemical and physical agent’s effects on genetic material. The recent studies refer to ionic rays, some diseases, chemical agents and some viruses can be causes modulating in the mitotic index (inhibition or stimulation). From the study of[9].
2. Aims of the Study
- The present study aimed to:1. Investigate of chromosomal analysis of type- 2 DM patients by using some parameters (MI, BI, and CA).2. Study of immunogenic state of type- 2 DM patients by measuring phagocytosis index and phagocytosis percent.
3. Materials and Methods
3.1. Samples Collection
- Between January to March 2010, 50 diagnosed cases of type-2 DM and 50 age- and sex-matched healthy control subjects were collected from specialized center for endocrinology and diabetes.
3.2. Lymphocyte Cultures and Cell Harvesting
- Peripheral blood samples were drawn by venipuncture into sodium-heparinized sterile syringe. For each donor, three blood cultures were set up. A 0.5-ml whole blood sample was added to a culture medium (5 ml) containing RPMI 1640 medium (pH 6.8–7.2), 15% fetal calf serum, 10 µg/ml phytohemagglutinin L (PHA-L), 0.5 mg/ml l-glutamine, and antibiotics (100 IU/ml penicillin, 100µg/ml streptomycin) for 24 h at 37℃.
3.3. Chromosomal Aberration Assay
- CA assay was performed for the entire incubation period of 72 h. Colcemid (10µg/ml) was added 70 h after initiation of all cultures. The cells were harvested and processed through treatments with a hypotonic solution (0.075M KCl) and fixative (3:1 methanol: glacial acetic acid). Chromosome slides were stained with 5% Giemsa solution (pH 6.8) for 2.5min, identified by the light microscope (Olympus, Japan) at oil emersion was used[10].
3.4. Phagocytosis Assay
- 2 ml of heparinized blood were centrifuged 3000 rpm (Boeco centrifuge U-320, Germany), A puffy layer (WBC) was gently isolated by glass Pasture pipette and suspended with 1 ml of (RPMI-1640), 100 µl of WBC solution (98% viability) have been incubated for one hour with 100 µl of heat killed Candida albicans suspension (1×10 3 cell/ml) at 37℃. 100 µl of the mixture (WBC and C. albicans) was spread on slide after completed dry fixed with absolute methanol and stained with 5% Giemsa solution (pH 6.8) for 2.5min, washed with PBS and slides were examined on 40X (Olympus, Japan) the percentage of phagocytosis was calculated using the following equation.
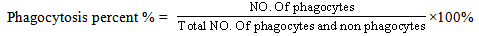
3.5. Blood Sugar Determination
- Blood sugar was determined according to manufacturer's instructions for diagnostic kit (Biolab, UK), the determination of serum glucose was conducted as the following steps.Pipette into 3 Test tubes
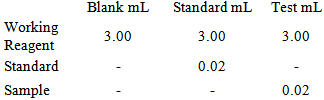 Mix well; incubate for 15 minutes at 37°C.Read the absorbency of the test and standard against blank at 500 nm wavelength. CalculationGlucose in mgs/dL= Absorbency of test / Absorbency of standard x 100.
Mix well; incubate for 15 minutes at 37°C.Read the absorbency of the test and standard against blank at 500 nm wavelength. CalculationGlucose in mgs/dL= Absorbency of test / Absorbency of standard x 100.3.6. Statistical Analyses
- SPSS program was used to analyze the results statistically, ANOVAI and multiple comparisons by using the least significant difference LSD was used at p≤005.
4. Result and Discussion
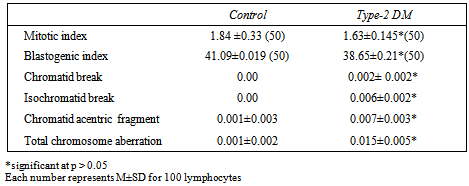
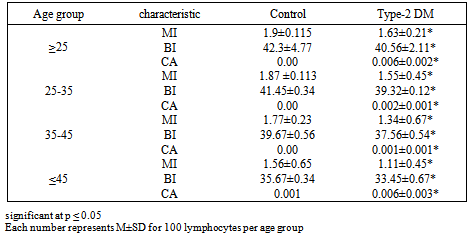
- Table 1. Shows the general characteristics of the study participants, the mean age of type-2DM patients was 36.12±8.11 years with body weight mean 67.43±5.45 and blood sugar level mean 223. 21± 15.13 mg/dl, 23 (46%) had a family history of type-2 diabetes and 5(10%) of patients had a family history of type-2 diabetes.Table 2 and figure 1 showed the frequencies of total CA, types of aberrations BI and MI in the two study groups there is a significant differences between patients and control group. Whilst table 3 refers some cytogenetic characteristics of two groups according to age, the MI, BI and CA were suffered from graduated reduced when increasing age.
|
|
|
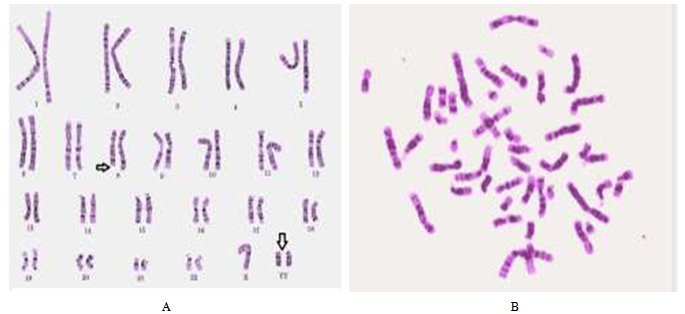 | Figure (1). A chromatide brakes in peripheral blood lymphocytes of type-2 DM, B metaphase of healthy one |
4.1. Phagocytosis Assay
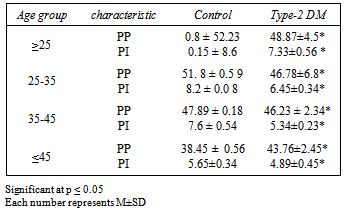
- The results in table 4 show the effects of type-2 DM on both phagocytoses index and percent according to age, we can see a clear reduction in both parameters with significant differences at P≤0.05, the reduction in PP in patients group reduced from 48.87 in age group ≥25 year to 43.76 in age group ≤45 the both effects (age and typ-2 DM) have the same effects on PMN leukocytes activity .
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML