-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2013; 2(4): 64-75
doi:10.5923/j.diabetes.20130204.02
Proportion of Anemia in Type 2 Diabetic Patients in Qena Governorate Case–Control Study: Clinical Correlates and Prognostic Significance
Hanan Mahmoud Fayed1, Abdel Rahman A Elsaied1, Mohammed Abdelrazek Alsenbesy2, Islam Ahmed Moubark1
1Clinical and Chemical Pathology Department, Qena Faculty of Medicine, South Valley University, Qena- Egypt
2Internal Medicine Department, Qena Faculty of Medicine, South Valley University, Qena- Egypt
Correspondence to: Hanan Mahmoud Fayed, Clinical and Chemical Pathology Department, Qena Faculty of Medicine, South Valley University, Qena- Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Diabetes mellitus is a major emerging clinical health problem in Egypt. Anemia is a common problem in diabetes.We aimed to explore anemia in type 2 diabetic patients (T2DM); determine relationship with disease duration, different stages of nephropathy and raise the awareness of anemia in T2DM in a cross-sectional survey of total of 100 T2DM patients and 50 healthy[age and sex matched] volunteers selected as control. All subjected to anthropometric parameters and blood pressure measurement, complete blood count, iron profile and spectrophotomteric measurement of routine kidney function and urine testing forcalculations ofurinary albumin creatinine ratio and estimated glomerular filtration rate. Irrespective of gender; T2DM had significantly higher proportion of anemia 63% (87.3 % in T2DM >45 years and 12.7 % in T2DM < 45 years), while 37% of T2DM (non-anemic with age > 45 years (94.6 %) and (5.4%) <45 years). Anemic T2DM had significantly lower eGFR (67.1 ± 3.0 vs. 87.9 ± 5.4 ml/min per 1.73 m2, p < 0.001) than non-anemic T2DM. Using Spearman correlation; T2DM had negative correlation between Hb versus FBG and urinary albumin. Our finding support the fact that anemia is a common complement to T2DM associated with poor glycemic control; that occurs earlier in diabetes particularly in T2DM with renal function impairment. Therefore, inclusion of anemia screening in the routine laboratory follow up required and anemic T2DM had to be referred to renal services for anemia management.
Keywords: Albumin/Creatinine Ratio, Anemia, BMI, Diabetes Type II, eGFR, Ferritin, HbA1c, Microalbuminuria
Cite this paper: Hanan Mahmoud Fayed, Abdel Rahman A Elsaied, Mohammed Abdelrazek Alsenbesy, Islam Ahmed Moubark, Proportion of Anemia in Type 2 Diabetic Patients in Qena Governorate Case–Control Study: Clinical Correlates and Prognostic Significance, International Journal of Diabetes Research, Vol. 2 No. 4, 2013, pp. 64-75. doi: 10.5923/j.diabetes.20130204.02.
Article Outline
1. Introduction
- Diabetes mellitus (DM) is a group of metabolic diseases characterized by hyper-glycemia resulting from defects in insulin secretion, insulin action, or both. The chronic hyperglycemia of DM is associated with long-term damage, dysfunction and failure of various organs, especially the eyes, kidneys, nerves, heart and blood vessels[1].DM is the leading cause of end-stage renal disease and significant proportions of patients with DM develop renal complications. Anemia is a key indicator of renal disease and potentially may contribute to the pathogenesis of diabetes complications[2]. Diabetes-related chronic hyperglycemia can lead to a hypoxic environment in the renal interstitium, which results in impaired production of erythropoietin by the peritubular fibroblasts and subsequent anemia[3]. Both deficiency and hypo responsiveness to erythropoietin contribute to anemia in DM patients with chronic kidney disease (CKD). The cause of erythropoietin deficiency is thought to be reduced renal mass with consequent depletion of the hormone. Possible causes of this erythropoietin hypo responsiveness include systemic inflammation and microvascular damage in the bone marrow[4]. Anemia in patients with DM might contribute to the pathogenesis and progression of cardiovascular disease and aggravate diabetic nephropathy and retinopathy. Anemia occurs earlier in patients with diabetic renal disease than in non-diabetic individuals with CKD[3]. In CKD, both absolute and relative iron deficiency are common. Absolute iron deficiency is defined as a depletion of tissue iron stores evidenced by a serum ferritin level < 100 ng/ml or a transferrin saturation of < 20%. Functional iron deficiency anemia is adequate tissue iron defined as a serum ferritin level > 100 ng/ml and a reduction in iron saturation. The latter is more common and is strongly associated with up regulation of inflammatory cytokines and impaired tissue responsiveness to erythropoietin, which can inhibit iron transport from tissue stores to erythroblasts[5]. Increased levels of inflammatory cytokines such as interleukin-6 enhance production and secretion of hepcidin, a hepatic protein that inhibits intestinal iron absorption and impairs iron transport from the reticuloendothelial system to bone marrow. In addition, erythropoietin, which normally enhances iron transport from macrophages to the blood stream, is impaired thereby exacerbating relative iron deficiency[6]. In addition, systemic inflammation, autonomic neuropathy and reduced red cell survival may also compound anemia in diabetes. While anemia may be considered a marker of diabetic kidney disease, reduced Hb levels, even within the normal range, identify diabetic patients with an increased risk of hospitalization and mortality. Anemia may also be significant in determining the outcome of heart failure and hypoxia-induced organ damage in patients with diabetes[7]. From the point of view of diabetic patients, anemia is an unknown complication of their disease. Raising awareness of anemia in diabetic patients is therefore of importance for the health care team[8], as anemia is associated with an increased risk of diabetic complications including nephropathy, retinopathy and macrovascular disease[9, 10]. It was suggest that; treatment of anemia will slow the progression of microvascular and macrovascular complications, including postural hypotension due to autonomic neuropathy, retinopathy and loss of renal function due to diabetic nephropathy. Furthermore; early diagnosis and treatment of anemia in diabetic patients may result in an improved quality of life and decreased morbidity and mortality[11].
2. Aim of the Work
- The objectives of this study were to:1) Explore proportion anemia in diabetic patients attending outpatient clinic of Qena university hospital. 2) Determine the relationship of disease duration and different stages of nephropathy in the development of anemia in these patients.3) Raise the awareness of anemia in diabetic patients which is of importance for the quality of health care management.
3. Study Design and Setting
- This was a cross-sectional case-control study in out-patient clinic of the internal medicine department; Qena university Hospital- South Valley University- Qena- Egypt.
4. Subjects and Methods
- ■ One hundred patients with T2DM included in the study; with different disease length duration[diagnosed based on the criteria of the American diabetes association, 2006 (ADA)[12]. An informed consent obtained from all participants. The control group were fifty healthy volunteers (matched for age and sex) and without any burdening or medical history.■ The study approved by the Ethical and Research committees in Qena university hospital.■ All cases were subjected to history taking including personal history, special habits, onset and duration of DM, associated medical diseases, DM treatment, glycemic control and current medication. Exclusion criteria● Analyses was limited to men and non-pregnant women aged >20 years.● Patients with thalassemia based on mean corpuscular volume (MCV) iron and total iron binding capacity (TIBC= Iron± UIBC) levels and with other systemic disorders that could result in anemia were excluded from the study. ● At the time of this study; clinics shared patient care with nephrology services were excluded. ● Results obtained outside the outpatient setting (e.g. patients in an emergency situation or hospitalized) were excluded.Physical examinationThrough clinical examination; including anthropometric measurements were obtained, including height, weight, peripheral pulsations, blood pressure, abdominal circumference and body mass index (BMI) was calculated in kilograms per square meter. Resting systolic blood pressures and diastolic blood pressures measured three times while the subjects seated, and the second and third measurements averaged. Hypertension defined as a blood pressure ≥140/90 mmHg or participant receiving current anti-hypertensive treatment. Participants completed standardized questionnaires that inquired about medical history, current medication, insulin doses, physical activity, alcohol and tobacco use, and family medical history. Both cases and controls were subjected to the following:-1. CBC and differentials performed on ethylenediamine tetra-acetic acid (EDTA) blood samples using Cell dyne 1800 cell counter (Abbott diagnostics, USA). To minimize the confounding effect of infection, subjects with a WBC count <4.0/109/l or >10.0/109/l were rechecked for the analysis and examined extensively for possible occult chronic infections. Any specimen with abnormal or atypical leukocytes was reanalyzed and excluded.2. Serum ferritin: was tested as instructed by the manufacturers using commercial automatedchemi-luminescent microparticle immune assay (CMIA) utilizing Chemi- Flex Technology (Architect i2000, Abbott diagnostics, USA). The Architect ferritin assay is a two-step immunoassay. In the first step, sample and anti-ferritin coated paramagnetic microparticles (mouse, monoclonal) are combined. Ferritin present in the sample binds to the anti-ferritin coated micro-particles. After washing, anti-ferritin acridinium labeled (rabbit, polyclonal) conjugate is added in the second step. Pre-Trigger and Trigger Solutions are then added to the reaction mixture; the resulting chemiluminescent reaction is measured as relative light units (RLUs). A direct relationship exists between the amount of ferritin in the sample and the RLUs detected by the Architect i optical system. The mean analytical sensitivity was calculated to be < 1 ng/mL.3. CRP rapid latex agglutination test for semi- quantitative determination of C-reactive protein in serum provided by (Biomed Diagnostics, EGY- CHEM for lab technology). Normal levels < 0.6 mg/dL.4. Spectrophotometric measurements of serum urea, creatinine, albumin, uric acid, calcium and Fasting blood glucose (FBS)[not for diagnostic purpose but to assess the blood sugar level and whether it is controlled or not] using Roche Cobas c311 automated chemistry analyzer (Roche diagnostics, Germany). 5. EDTA anti-coagulated sample used for hemoglobin A1c (HbA1c) measurements using automated cation-exchange high-performance liquid chromatography (HPLC) provided by Bio-Rad Laboratories D-10 (Bio-Rad Laboratories, USA). Results have been reported as percent (100 × HbA1c/total Hb). Reference range = 3.8–18.5%6. Inorganic phosphorus level measured by Fiske-Subbarow method quantitative colorimetric end point assay according to the kit manufacturer's instructions provided by Quimica Clinica Aplicada S.A. Normal values: 2.5-5.5 mg/dl.7. Spot urine sample for estimation of urinary albumin/ creatinine ratio (UACR): morning clean mid-stream samples collected and centrifuged at 3000 rpm for 10 min, then divided into two parts:-a) One part was diluted 1:50 with distilled water and used for spectrophotometric estimation of urine creatinine by Roche Cobas c311 automated chemistry analyzer (Roche diagnostics, Germany).b) Another part used for detection of protein in urine by immune- turbidimetric method in random urine samples (and confirmed by 24-h collections of sterile urine). UACR used to classify diabetic patients according to the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines; into non-albuminuria <30 mg/g),micro-albuminuria (30–300 mg/g, or macro-albuminuria (>300 mg/g) [13].8. Urine 24 hour sample collection for estimation of microalbuminuria and urinary albumin execration rate (AER) (prior to 24h- urine collection; sample first screened for signs of infection using a dipstick (Multistix® 10SG reagent strips, Siemens Diagnostics, Poland) and urine sediment examined microscopically and samples infected, leukocyturia and/or eryhthrocyturia were discarded) samples then categorized as normo- micro- and macroalbuminuria when (AER of < 20 μg/ min), 20–200 μg/ min and > 200 μg / min, respectively, and confirmed twice[14]. 9. Estimated glomerular filtration rate (eGFR) as an indicator of renal function, estimated from serum creatinine using a formula that accounts for the influence of age and gender on creatinine production; GFR = 186.3 × (serum creatinine-1.154) × (age -0.203)×1.212(if black)× 0.742 (if female). GFR expressed in ml/min/1.73 m2[15], and patients considered to have chronic kidney disease when the estimated GFR was < 60 ml/min per 1.73 m2[16].The presence of anemia was defined by an Hb level less than 13 g/dl in men and less than 12 g/dl in women, which is a gender-specific definition established by the WHO. To focus on clinically significant and potentially treatable anemia, we used a more strict definition (Hb level less than 12 g/dL for men and less than 11 g/dL for women). A second definition of anemia used based upon the suggested threshold of 11 g ⁄ dl for the initiation of treatment for anemia in chronic kidney disease using erythropoietin[17]. Iron deficiency anemia was defined as the presence of both anemia and a plasma ferritin concentration less than 15 ng/ml WHO[18]. Iron overload was defined as ferritin > 300 ng/ml for men and > 200 ng/ml for women[19]. Statistical analysis:Data analysis by IBM computer using (statistical program for social science) SPSS version 12 as follows: ● Description of quantitative variables as mean, SD and range ● Description of qualitative variables as number and percentage ● Chi-square test used to compare qualitative variables between groups.● Unpaired t-test used for comparison of quantities variables, in parametric data (SD<50%) of mean. ● One way ANOVA test was used to compare more than two groups as regard quantitative variable. ● Spearman correlation (r) was used to assess the strength of the correlations between peripheral total and differential leukocyte counts, albuminuria and hemoglobin positively or inversely with metabolic parameters in patient's group.● Logistic regression model used to find out the most significant independent predictors for dependent variable ● P value >0.05 insignificant, P<0.05 significant and P<0.01 highly significant
5. Results
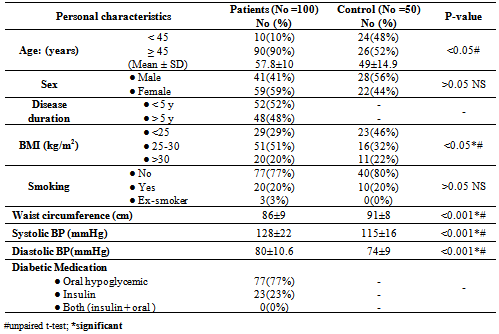
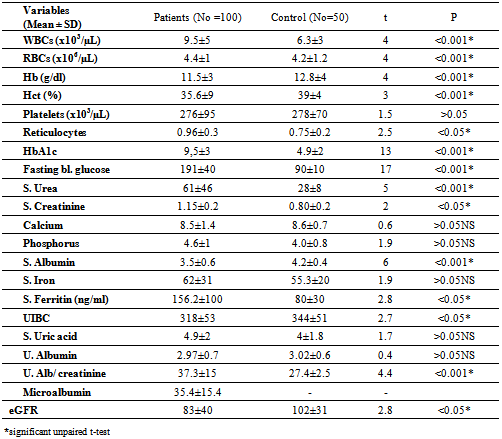
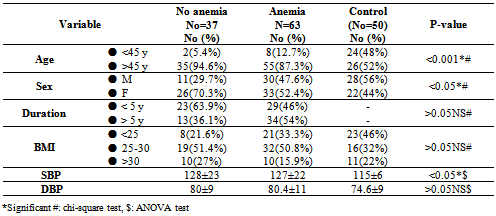
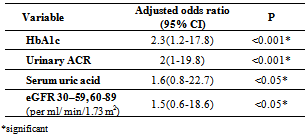
- Table 1 showed the demographic characteristics of all the subjects studied. Subjects were divided into 2 groups: Group 1: One hundred T2DM; 41(41%) males and 59(59%) females with mean age of 57.8±10 years, 10 (10%) < 45years and 90 (90%) > 45 years old.Group 2: Fifty apparently healthy non diabetic subjects living in the same environment/city; 28(56%) males and 22(44%) females mean age of 49±14.9 years, 24(48%) of control < 45 years and 26(52%) >45 years old.The diabetic patients were significantly older with higher BMI (p <0.05), blood pressure (p <0.001) and lower waist circumference (p < 0.001) than the non-diabetic controls.Table 2 showed the laboratory characteristics of all the studied subjects. Irrespective of gender, diabetic patients had significantly lower Hb level and higher prevalence of kidney dysfunction and lower serum albumin compared with the non-diabetic controls. In additions; T2DM had significant higher WBCs count, fasting serum glucose and HbA1c. Table 3 showed the demographic and clinical characteristics of anemic and non-anemic T2DM patients compared to control. Anemic T2DM patients had a significant higher age than non-anemic, and anemia affects females more than males and both anemic and non-anemic T2DM have higher SBP compared to controls.
|
|
|
|
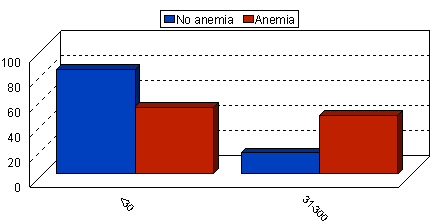 | Figure 1. Normo-microalbuminuria frequency in anemic and non-anemic type 2 diabetic patients |
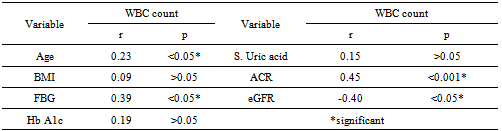
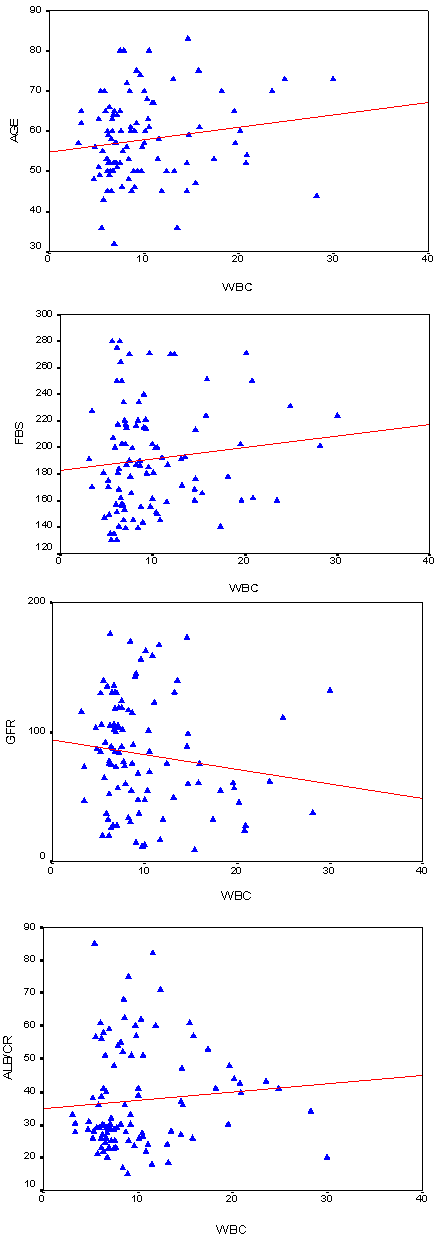 | Figure 2. Spearman Correlation Test between WBCs versus Age, FBG,eGFR and Urine Albumin/Creatinine Ratio among T2DM Patients |
|
|
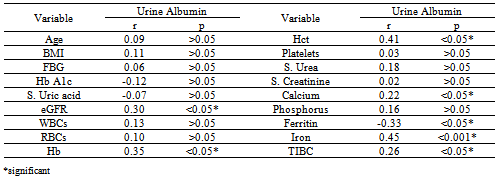
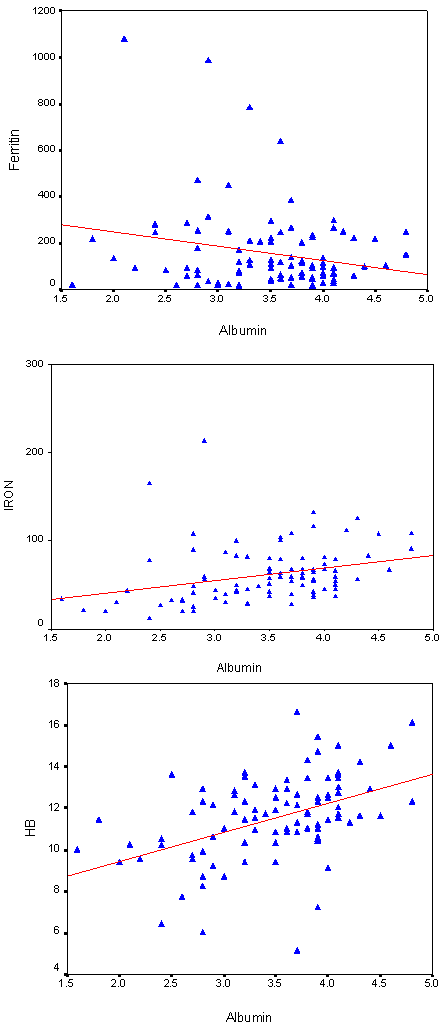 | Figure 3. Spearman Correlation Test between Urine Albumin versus Ferritin, Iron and Hb among T2DM Patients |
|
|
|
6. Discussion
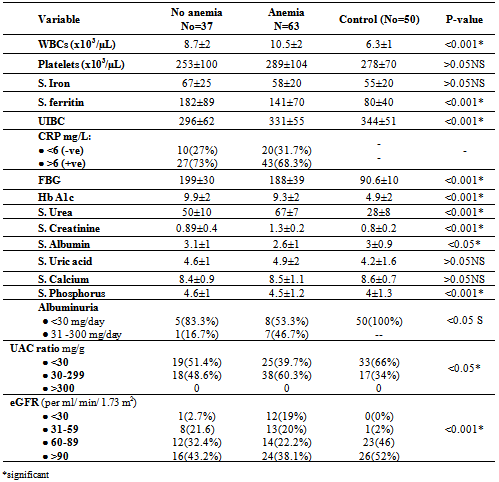
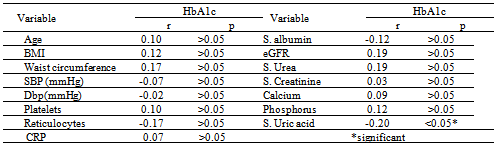
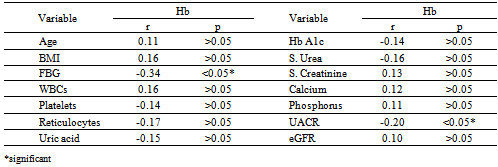
- DM is a major emerging clinical and public health problem in Egypt. The total prevalence of DM in Egypt was estimated in 1995 by 9.9%, and the predicted prevalence by the year 2025 will reach 13.3%[20]. It is estimated that in 2025, Egypt may be among the 10 "leading" countries in the world in terms of the number of people with DM[21]. DM is one of the most common causes of CKD. Although patients with DM are regularly monitored for a variety of complications, such as neuropathy, nephropathy and retinopathy; Hb concentrations frequently are not routinely assessed. Interestingly, reductions in Hb often occur before the onset of overt diabetic nephropathy[22]. In the primary care setting in the clinics, assessment of hematological parameters is no part of routine diabetic evaluation. This study shown that anemia was prevalent in T2DM subjects, after exclusion of iron deficiency anemia and other systemic disorders and irrespective of gender; T2DM patients with anemia had the lowest kidney function compared with patients without anemia. In our study in T2DM patients; the prevalence of anemia was (63%) where 55(87.3%) >45 years and 8(12.7%) <45 years. while (37%) of T2DM patients were non-anemic with age 35(94.6 %) >45 years and 2 (5.4%) <45 years. Anemic T2DM patients had significant lower serum albumin compared to non-anemic and controls; 25(39.7%) had normoalbuminuria and 38 (60.3%) had microalbuminiura compared to non-anemic patients 19 (51.4%) had normoalbuminuria and 18(48.6%) had microalbuminiura. In T2DM; anemic patients 38(60.3%) and non-anemic 28(75.6%) had eGFR > 60 ml/min per 1.73 m2, whereas 9(24.3%) non-anemic T2DM patients and 25 (39.6%) anemic patients had eGFR < 60 ml/min per 1.73 m2. The prevalence of microalbuminiura in all patients was 56%. The majority of the anemic T2DM patient had UACR 30-299 mg/g 60.3%, and 39.7% had UACR <30 mg/g. Similarly; most anemic T2DM patient 60.3% had eGFR >60 ml/min per 1.73 m2, only 20.6% and 19% having eGFR levels of 31 to 59 and <30 ml/min per 1.73 m2, respectively. Those with higher levels of UACR and lower eGFR were older with longer duration of DM; but no significant difference between anemic and non-anemic T2DM patients regarding blood pressure.Also there were a significant difference between T2DM patients and control group regarding WBCs count (9.5±5 vs. 6.3±3) (p <0.001), RBCs (4.4±1 vs. 4.2±1.2) (p <0.001), Hb (11.5±3 vs. 12.8±4 g/dl) (p <0.001) and Hct (35.6±9 vs. 39±4) (p <0.001), s. ferritin (156.2±100 vs. 80±30) (p <0.05), UIBC (318±53 vs. 344±51) (p <0.05), FBG (191±40 vs. 90±10) (p <0.001), HbA1c (9.5±3 vs. 4.9±2) (p<0.001), serum urea (61±46 vs. 28±8) (p <0.001), serum creatinine (1.15±0.2 vs. 0.80±0.2) (p <0.05), serum albumin (3.5±0.6 vs. 4.2±0.4) (p <0.001), urine albumin to creatinine ratio (37.3±15 vs. 27±2.5) (p <0.001), and eGFR (83±40 vs. 102±31 ml/min per 1.73 m2, p <0.05). These results were consistent with the previous literature;[23-34]. In our study; among diabetic patients there was a significant negative correlation between WBCs count (as a parameter of systemic inflammation) versus eGFR and there were a significant positive correlations between WBCs count versus age, FBG and UACR; This in accordance to[35]. WBCs count and it is correlated with FBG, UACR. And he found that peripheral total WBC, monocyte and neutrophil counts were increased in parallel with the advancement of diabetic nephropathy but in contrast; the lymphocyte count decreased. And he speculated that leptin might be involved in increased leukocyte counts.In our study; among T2DM there was a significant negative correlation between urinary albumins versus s. ferritin (r value -0.33, P < 0.05) while a significant positive correlations found versus eGFR (r value 0.30, P < 0.05), Hb (r value 0. 35, P < 0.05), Hct (r value 0.41, P < 0.05), s. calcium (r value 0.22, P < 0.05), s. iron (r value 0.45, P < 0.05) and s. TIBC (r value 0.26, P < 0.05).In our study there was a significant negative correlation between HbA1c and serum uric acid (r value -0.20, P < 0.05). This was in agreement with[36, 37]. Although Serum ferritin is the most commonly used marker of stored body iron However, serum ferritin was positively and hemoglobin was negatively associated by inflammation and infection (38). Moreover; inflammation was postulated to be involved in the physiopathological mechanisms behind metabolic syndrome and diabetes [39-41], by promoting both insulin resistance and increased oxidative stress[42, 43].In our study; anemic T2DM patients had a significant lower serum ferritin compared to non anemic T2DM patients but a significantly higher than of controls. This was in agreement with other studies[44-47]. In our study; anemic T2DM patients had a significant lower HbA1c than non-anemic T2DM; this was in agreement with[48].In our study; T2DM patients had significant negative correlation between Hb versus FBG and albuminuria; this was in agreement with[10, 33, and 49]. In our study; reductions in kidney function and dysglycemia were associated with increased odds of micro- macro-albuminuria; this was in agreement with[50, 51].
7. Limitations
- This study has got many limitations; first: small number of the studied diabetic patients; community-based screening is a must for proper estimation of the prevalence of anemia among diabetics. Second: the cross-sectional design; which prevents us from making inferences about the directionality of the associations. Third: dietary pattern assessment; especially for iron intake was not considered in our study. Although there is increasing evidence to suggest that iron may influence glucose metabolism, it is possible that glucose metabolism may also influence body iron stores. Fourth: antihypertensive therapy not considered in our study.
8. Conclusions
- In spite of the limitation of the study the results were consistent with some previously reported results of anemia is a common complement to diabetes (and its association with increased iron stores); which occurs earlier in T2DM particularly in patients with renal function impairment. Therefore, inclusion of anemia screening in the routine laboratory follow up in diabetic patient is required and anemic patients should be referred to renal care services for proper anemia management together with nutrition specialist for diet and lifestyle modification. The best possible glycaemic control and antihypertensive treatment; including inhibition of the renal angiotensin aldosterone system are important in preventing and ameliorating the course of normo- and micro-albuminuria.
Abbreviations
- DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; FBG: Fasting blood glucose; UAC: Urine albumin/Creatinine ratio; SBP: Systolic blood pressure
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML