-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2013; 2(3): 50-55
doi:10.5923/j.diabetes.20130203.03
Macro- and Microvascular Complications of Diabetes Induced by High-Fat Diet and Low-Dose Streptozotocin Injection in Rats Model
Doan Viet Binh, Nguyen Thi Kim Dung, Le Thi Bich Thao, Nguyen Bich Nhi, Phan Van Chi
Institute of Biotechnology (IBT), Vietnam Academy of Science& Technology, Hanoi, Vietnam (VAST)
Correspondence to: Phan Van Chi, Institute of Biotechnology (IBT), Vietnam Academy of Science& Technology, Hanoi, Vietnam (VAST).
| Email: | 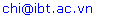 |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this study, the development of micro- and marcrovascular diabetic complications induced by high-fat diet and low-dose streptozotocin injection in rats model during 24 weeks was characterized by the increasing their growth rate/body weight, histological changes in the kidney, aorta and biochemical parameters. It was shown that all of the high-fat diet plus STZ injection rats exhibited remarkable lesions and plaque in their aorta representing damage to large blood vessels. Rats suffered from hyperglycemia in whole studying duration exhibited global glomerulosclerosis, hyaline arteriosclerosis and glomerular nodule, which are comparable with characteristics observed in late stage of human nephropathy. The rest diabetic rats demonstrated features of mild nephropathy corresponding with an early stage of kidney disease. The profiling of biochemical parameters indicated that blood glucose, lipids, HbA1c and urine microalbumin were much higher in high-fat diet plus STZ injection rats than that in controls.
Keywords: Diabetes, High-fat Diet and Low-dose Streptozotocin, Micro- and Macrovascular Complications
Cite this paper: Doan Viet Binh, Nguyen Thi Kim Dung, Le Thi Bich Thao, Nguyen Bich Nhi, Phan Van Chi, Macro- and Microvascular Complications of Diabetes Induced by High-Fat Diet and Low-Dose Streptozotocin Injection in Rats Model, International Journal of Diabetes Research, Vol. 2 No. 3, 2013, pp. 50-55. doi: 10.5923/j.diabetes.20130203.03.
Article Outline
1. Introduction
- Nowadays, diabetes trends to spread throughout the world in both developed and developing countries. A recent review showed that there would be a 69% increase in numbers of adults with diabetes in developing countries between 2010 and 2030[16]. Many patients unknowingly have an ongoing disease while the progression from prediabetic state to overt diabetes takes years. In fact, the disease can lead to the development of micro- and macrovascular complications, which contribute greatly to its morbidity and mortality. An obvious relationship between type 2 diabetes mellitus (T2DM) and cardiovascular disease (CDV) has been shown [1]. A meta-analysis of retrospective cohort studies on the risk of cardiovascular adverse effects in patients with type 2 diabetes treated with pioglitazone compared to rosiglitazone has indicated that the later was associated with an increased risk of myocardial infarction, heart failure, and all-cause mortality in diabetic patients[2].Diabetic complications have been already known, including increased thickness of the intima of thoracic aorta[11], cardiac autonomic neuropathy[19], foot ulcers[20], and chronic kidney disease[4]. It is interesting that the relationships between HbA1c and both fibrinogen and HDL - cholesterol and between HDL-cholesterol and fibrinogen were significant only in coronary artery disease[12]. Diabetic complications have been studied on animal model in order to study the pathological mechanisms[7], to identify new biomarkers for early diagnosis[15] and to test new medicine for therapy of that disease[13]. The advantages of research of diabetic complications on animal models include a relatively short time span of experiment, genetically homogeneity, possibility of tightly control of dietary intake, supply of tissues that are difficult to obtain from human subjects[15]. Until now, the most commonly used model of insulin independent diabetes is high-fat diet and low-dose streptozotocin (STZ) injection[17, 22, 23]. It is easily attainable and inexpensive. Ratna et al.[14] presented a non-genetic rat model of type 2 diabetes mellitus with nephropathy. As the duration of diabetes was also relatively short in this study, only mild changes in kidney were noticeable and there were no other assessments of vascular function. Therefore, longer studies are needed to determine if the induced diabetes would cause remarkable vascular and renal complications. In this article we report about result of our research on a model of diabetic rats, which was induced by high-fat feeding and low dose injection of streptozotoxin. Twenty four weeks after induction of diabetes, remarkable damage was detected in kidney and aortic arch of the rats. Both histological and biochemical analysis showed that the rat model is suitable for investigating diabetic complications.
2. Materials and Methods
- In our experiments, all the reagents were analytical grade and used without further purification. Streptozotoxin (STZ), sodium cholate hydrate, sodium citrate were purchased from Sigma-Aldrich (Sigma-Aldrich Co., USA), cholesterol from Kanto Chemical (Kanto Chemical Co., Inc., Tokyo, Japan), sucrose and casein from Merck (Germany).
2.1. Experiment Design
- Wistar male rats (170-190 g) were randomly divided into 2 groups: control group and high-fat diet plus STZ injection group. All rats were first provided with normal pellet diet and water ad libitum, one week prior to the dietary manipulation. Then, the control group was fed with regular pellet, while high-fat diet plus STZ injection group was fed with high-fat diet for 6 weeks. The regular pellet consisting of 5% fat, 45-55 % carbohydrate, 23 % protein, with total caloric value of about 3,100 kcal/kg, was supplied from National Institute of Hygiene and Epidemiology, Hanoi. High-fat diet contained 52.5 % regular pellet, 35 % lard, 6.25 % sucrose, 5 % casein, 1% cholesterol, 0.25 % choline (60 % calories from fat, and total caloric value was about 5,200 kcal/kg).Rats of high-fat diet plus STZ injection group were then injected with a low dose of STZ (35 mg/kg) in a dose volume of 0.5 ml, intraperitoneal (i.p.), while rats of the control group were given vehicle-sodium citrate buffer (pH 4.5). After one and six weeks of STZ injection, blood glucose was measured. Only rats with blood glucose <16.7 mmol/L were injected again with a higher dose of STZ (40 mg/kg). All the rats were then allowed to continue to feed on their respective diets and closely monitored for further 24 weeks until the end of the study. Rat body weights were recorded every month.
2.2. Histological and Biochemical Analysis
- Rats were sacrificed at 24th week after induction of diabetes. Segments (5 mm long) of the aortic arch beginning from the junction with the heart, and kidneys were excised, then fixed in 10% formalin solution for routine paraffin embedment. Representative sections (4 µm thick) were prepared and stained with hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) for microscopic evaluation. The sections were then examined by differential interference contrast (DIC) microscopy on the Olympus BX51 (Olympus Co., Japan) for the degree of vascular and renal injuries. Before tissue preparation, blood was collected from retro-orbital plexus of the rats under light ether anesthesia using capillary tubes and eppendorf tubes containing heparin (20 µl, 200 IU ml−1, 0.1ml heparin/ml blood) or ethylenediaminetetraacetic acid (EDTA) (2.0 mg/ml). The serum was separated by centrifugation (5 min, 3,000 rpm) and was analyzed for glucose, triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-cholesterol), low-density lipoprotein cholesterol (LDL-cholesterol), and HbA1c by using a Cobas 6000 Analyzer (Roche, Germany). The kits for the analysis were supplied from Roche Diagnostics (Roche Diagnostics GmbH, D-68298 Mannheim). HbA1c was determined on blood samples obtained at the end study period. At the time of sacrifice, urine was also collected from the bladder and microalbumin level was measured using the Cobas 6000 Analyzer.
2.3. Statistics
- Statistical analysis of the difference between experimental and control groups was carried out using the Student’s t-test.
3. Result
3.1. Body Weight
- Experimental rat body weights during the observation period are shown in figure 1. In the study duration, both groups of rats exhibited a continuously growth but the high-fat diet plus STZ injection rats were much heavier than the controls. This difference in body weight between diabetic and non-diabetic animals was statistically significant (p<0.001).
 | Figure 1. Comparison of growth rate of the experimental rats in the study period. High-fat diet plus STZ injection rats were heavier than controls throughout the study period |
3.2. Histological Changes in the Kidney
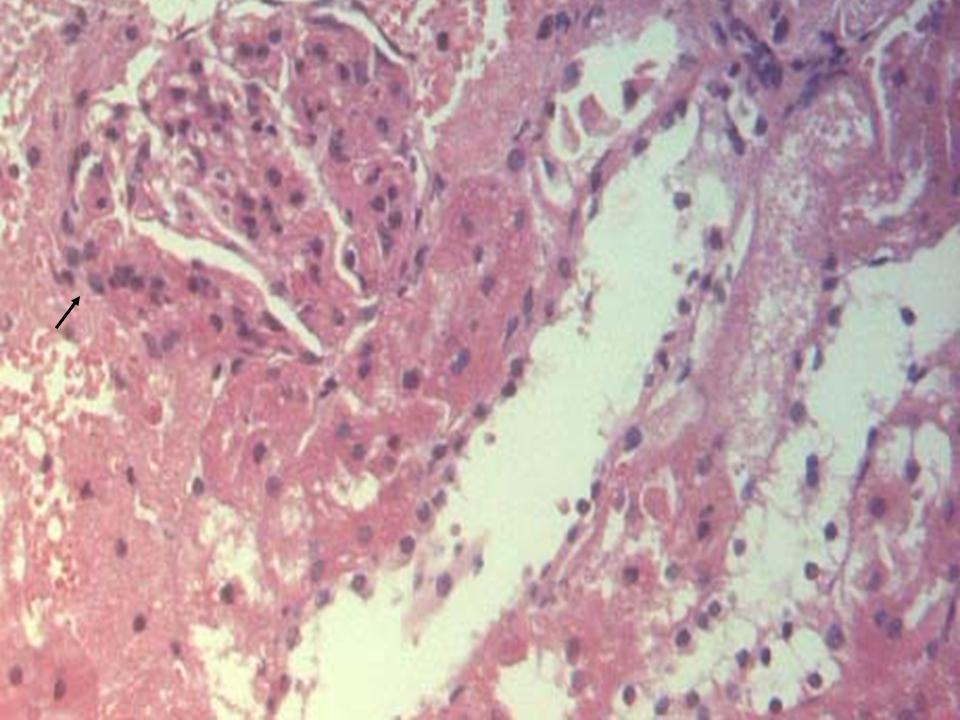
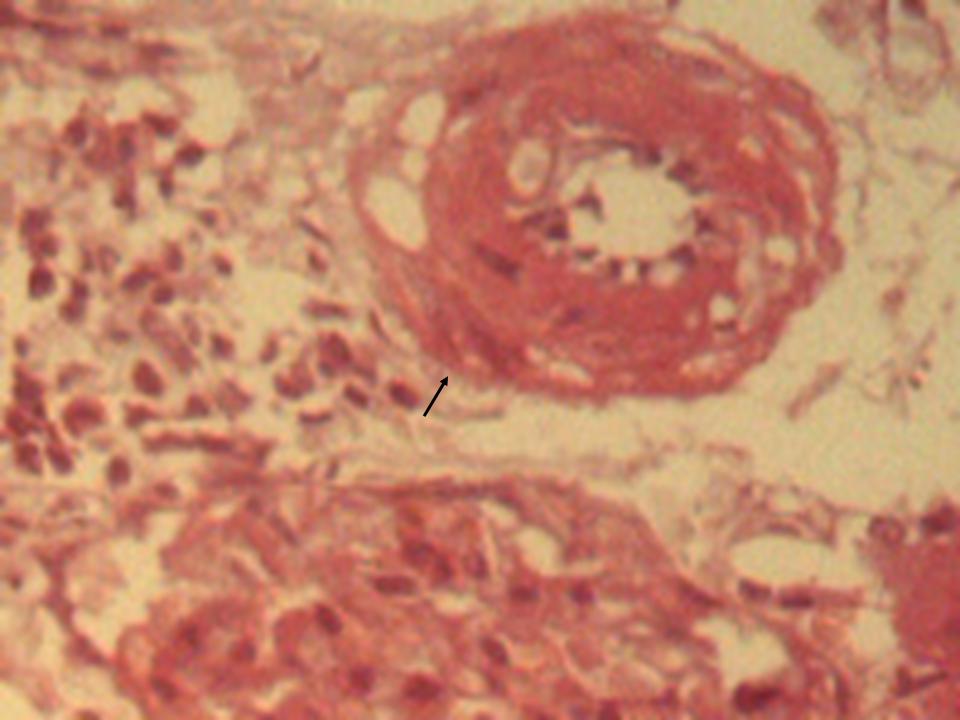
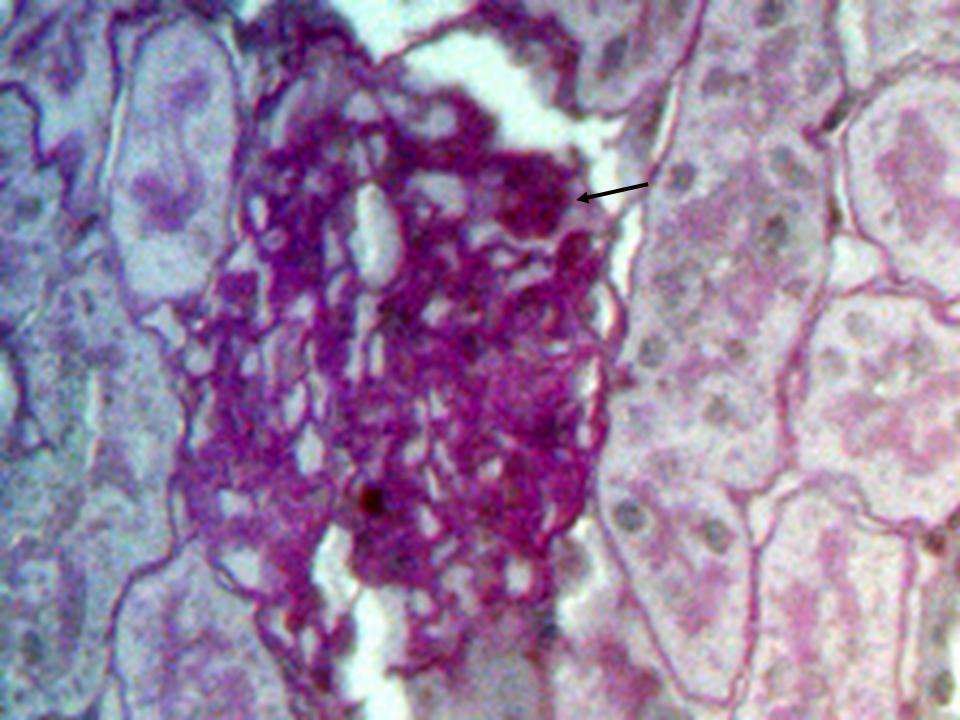
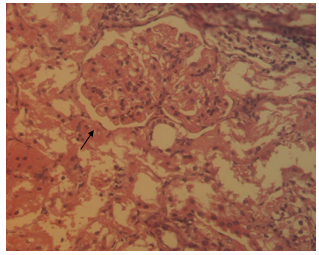
- The histological findings in kidney of all the high-fat diet plus STZ injection rats showed features of glomerulopathy, but there was a difference between the only once and the repeated STZ injected rats from case to case. The former rats exhibited predominant features such as focal segmental glomerulosclerosis (Figure 2), global glomerulosclerosis with several tubules with intraluminal hyaline casts, atrophic glomeruli and hyaline arteriosclerosis (Figure 3). In some cases, glomerular nodule was observed (Figure 4), while other cases demonstrated glomerular hypertrophy, increased mesangial matrix, and glomerular basement membrane thickening (Figure 5). In the control rats, normal renal tubular architecture and normal glomeruli were observed.
3.3. Histological Changes in the Aorta
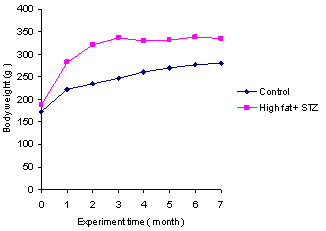
- Histological analysis of the aortic arch showed that all rats fed the high-fat diet plus STZ injection developed early atherosclerotic lesions, extensive aortic fatty streaks, and advanced atherosclerotic plaques (Figure.6).
 | Figure 6. An atherosclerotic lesion of a high-fat diet plus STZ injection rat with small lipid core (arrow head) and foam cell (black arrow). H & E stained, magnification ×400 |
3.4. Biochemical Parameters
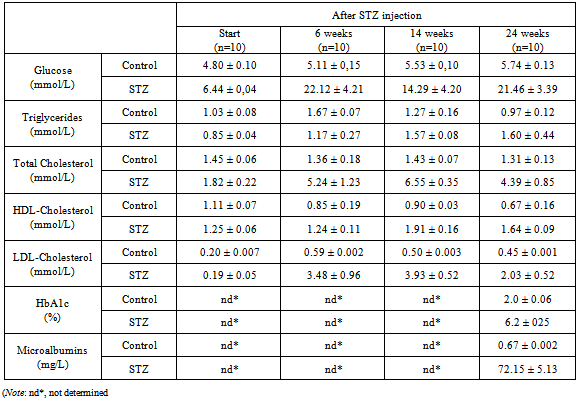
- The profiling of biochemical parameters, including blood glucose, triglycerides, total cholesterol, HDL-cholesterol, LDL-cholesterol, HbA1c, urine microalbumins of the experimental rats from beginning to the end of the study period was presented in Table 1.In general, the data in table 1 showed that all the STZ injected had higher fasting blood glucose as compared with that of the control. Glucose levels of control rats were slightly changed in the study period. It’s interesting that fasting blood glucose levels of the high-fat diet plus STZ injection rats increased rapidly as compared with that of the control and reached 22.12 ± 4.21 mmol/L after next 6 weeks, 14.29 ± 4.20 mmol/L after 14 weeks. From that time, repeated STZ injection according to the experiment design (only for the rats with glucose <16.7 mmol/L) increased the glucose level of this group up to 21.46 ± 3.39 mmol/L after 24 weeks. This agreed with the published study showed that a better and stable animal model of type 2 diabetes mellitus by high-fat diet with multiple low-dose STZ injection[23]. The levels of triglyceride and HDL-cholesterol of high-fat diet plus STZ injection rats were increased slightly at the end of the experiment, while these parameters of the control rats were decreased. Meanwhile, the levels of total cholesterol and LDL-cholesterol of high-fat diet plus STZ injection rats were many times higher than that of control rats (p<0.001).It should be noted also that, at the end of experiment period (24th week after STZ injection), the concentration of blood HbA1c of the high fat diet plus STZ injection group rats were 6.20±0.25 %, much higher than that of the control group (2.00±0.06 %) (Table 1). Beside, the measured microalbumins in the urine of high-fat diet plus STZ injection rats were 72.15 ± 5.13 mg/L, whereas the measured levels of control rats were only 0.67 ± 0.002 mg/L.
4. Discussion
- Diabetes complications have been classified as microvascular and macrovascular representing damage from small and large blood vessels to kidney. Risk factors for vascular damages are chronic hyperglycaemia, hypercholesterolemia, hyperlipidaemia, and hypertension... Each risk factor, when considered alone, increases vascular disease risk, but more importantly, in combination they could provide an additive or even synergistic effect[3].Kidney involvement in a nongenetic rat of type 2 diabetes induced by high-fat diet and low dose STZ injection has been shown[4]. Nephropathy is one form of microvascular complications. The early pathogenesis of diabeticnephropathy begins with hyperglycemia causing glomerular hyperfiltration, which results in glomerular hypertrophy and glomerular basement membrane thickening. This damage allows proteins in the blood (such as albumin) to leak into the urine, causing increased excretion of protein (proteinuria). Therefore, one of the early signs of diabetic nephropathy is the presence of microalbuminuria. Progressive proteinuria and glomerulosclerosis leading to formation of nodules In our experiment, the high-fat diet plus STZ injection rats exhibited hyperglycemia,hypercholesterolemia and demonstrated features of differentglomerulopathy. Rats suffered from hyperglycemia in whole 24 weeks of study duration showed global glomerulosclerosis, hyaline arteriosclerosis and glomerular nodule, which are comparable with characteristics observed in late stage of human nephropathy[7, 8]. Rats, whose hyperglycemia extended in brief duration and needed repeated STZ injections demonstrated features of mild nephropathy corresponding with an early stage of kidney disease. This finding showed that the duration of hyperglycemia plays an important role in pathogenesis of diabetic nephropathy and agreed with results documented by groups of Matsumae at al. and Suzuki at al.[10, 18].
|
5. Conclusions
- The development of micro- and marcrovascular diabetic complications induced by high-fat diet and low-dose streptozotocin injection in rats model during 24 weeks was characterized not only by biochemical parameters, but also by exhibition of remarkable lesions and plaque in their aorta representing damage to large blood vessels and global glomerulosclerosis, hyaline arteriosclerosis and glomerular nodule.
ACKNOWLEDGEMENTS
- This work was funded by the Research Project 03/ 2011/ PTNTĐ/ HĐ-ĐTĐL and was carried out at Key Lab for Gene Technology, Institute of Biotechnology, Vietnam Academy of Science & Technology, Hanoi.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML