-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2013; 2(3): 45-49
doi:10.5923/j.diabetes.20130203.02
Melatonin Supplementation Improves Glycemic Control While Lowering Oxidative Stress in Type 2 Diabetes
Carmine R. Grieco1, Sheri R. Colberg2, C. Thomas Somma3, Andrew G. Thompson2, Aaron I. Vinik4
1Department of Education, Glenville State College, Glenville, WV, United States
2Department of Human Movement Sciences, Old Dominion University, Norfolk, VA, United States
3School of Medical Laboratory and Radiation Sciences, Old Dominion University, Norfolk, VA, United States
4Strelitz Diabetes Research Institute, Eastern Virginia Medical School, Norfolk, VA, United States
Correspondence to: Carmine R. Grieco, Department of Education, Glenville State College, Glenville, WV, United States.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
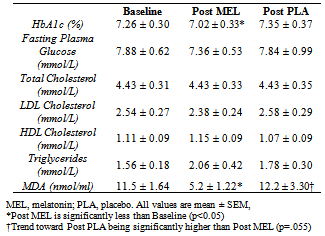
The purpose of this investigation was to evaluate the effect of melatonin on glycemic control and oxidative stress (OS) in adults with type 2 diabetes (T2D). Fourteen subjects with T2D (10 female, 4 male; 52.5 ± 5.0 years) were randomly assigned to melatonin (MEL) or placebo groups (PLA) for 42 days, in a crossover design. Subjects ingested 10 mg of MEL or an identical placebo (PLA) 30 minutes prior to sleep. Fasting blood draws occurred at baseline, 42 days, and 84 days. Plasma malondialdehyde, a marker of OS, significantly decreased on MEL (-6.25±2.10 nmol/ml) compared to PLA (0.72±3.30, p=0.028). The change in hemoglobin A1c showed a total improvement of 0.33% following MEL supplementation compared to PLA (-0.24±0.23 % for MEL vs. 0.09±0.21 % for PLA, p=0.01), although no significant changes were noted in fasting plasma glucose or lipid levels. Daily melatonin may diminish OS and enhance glycemic control in adults with T2D.
Keywords: Melatonin, Oxidative Stress, Type 2 Diabetes, Hemoglobin A1c
Cite this paper: Carmine R. Grieco, Sheri R. Colberg, C. Thomas Somma, Andrew G. Thompson, Aaron I. Vinik, Melatonin Supplementation Improves Glycemic Control While Lowering Oxidative Stress in Type 2 Diabetes, International Journal of Diabetes Research, Vol. 2 No. 3, 2013, pp. 45-49. doi: 10.5923/j.diabetes.20130203.02.
Article Outline
1. Introduction
- It is commonly accepted that oxidative stress plays a significant role in the pathogenesis of type 2 diabetes mellitus (T2D)[1] and, according to the “unifying hypothesis” of Brownlee may, in fact, be the single most critical factor contributing to complications of T2D[2]. Oxidative stress can be broadly described as an imbalance between reactive oxygen species (ROS) production and the cellular ability to reduce these potentially harmful substances through antioxidant defenses. At physiological levels ROS are important cellular signaling molecules and a normal byproduct of cellular metabolism. Under optimal conditions ROS molecules are rigidly controlled by endogenous antioxidant defenses, effectively nullifying any deleterious effect. T2D, however, represents a disease state in which there is an imbalance between antioxidative resources and oxidant (free radical) production, largely driven by hyperglycemia that results in a state of oxidative stress. Melatonin is the primary secretory product of the pineal gland and is most frequently associated with circadian rhythms and the sleep cycle[3]. Melatonin, however, is also a potent and unique antioxidant[4] that has demonstrated an ameliorative effect upon oxidative stress in human and animal models of T2D[5, 6, 7, 8]. Given the localization of its receptors in the suprachiasmatic nucleus (SCN) of the hypothalamus, the body’s primary circadian pacemaker, melatonin plays a major role in modulating both the sleep-wake cycle and circadian rhythms in humans[9]. The hypothalamus is also the dominant brain region responsible for sensing and responding to blood glucose levels[10] and controlling their circadian rhythm[11, 12]. However, hypothalamic activity is reduced[13] and melatonin secretion frequently attenuated and phase-delayed in individuals with T2D[14]. This melatonin deficit may contribute to nocturnal elevations in hepatic glucose output[14]. Deficits in hypothalamic activity, specifically SCN output, may directly contribute to the pathophysiological development and exacerbation of T2D[13, 14] via autonomic dysfunction and oxidative stress. As the main synchronizer of the body’s biological clock, melatonin has demonstrated a restorative ability on SCN output[15] and sleep, which may improve autonomic nervous system (ANS) balance[16] oxidative stress[6, 17, 18] and glycemic control[14, 19] in T2D.A plethora of studies using animal models and human cell lines provide intriguing evidence of the effectiveness of melatonin at improving glycemic control[20, 21], increasing endogenous antioxidant defenses[22, 23, 24] and decreasing oxidative stress[25, 26, 27, 28, 29]. To date, however, only a limited number of studies have investigated the potential role for melatonin in improving oxidative balance and glucose regulation in-vivo in T2D. Thus, the purpose of this investigation was to evaluate the effect of a commercially available preparation of melatonin on both oxidative stress and glycemic control in adults with T2D.
2. Materials and Methods
- Fourteen subjects (10 female, 4 male; 52.5 ± 5.0 years) with uncomplicated T2D (duration of 7.4 ± 5.3 years) were randomly assigned to a melatonin group (MEL) or placebo group (PLA) for 42 days, followed by 42 days in the alternate group in a crossover design. Exclusionary criteria included sleep apnea or other sleep disorders, congestive heart failure, myocardial infarction, arrhythmia or any cardiovascular event in the previous year, liver disease, kidney disease, orthostatic hypotension, or diagnosis of previous or current psychiatric disorder. Ten of the subjects were currently taking oral glucose-lowering drugs and four subjects were diet only. Each subject maintained their treatment regimen for the length of the investigation. During each supplementation period, subjects ingested either 10 mg of commercially available melatonin in capsule form (Quality Supplement and Vitamins, Inc., Ft. Lauderdale, FL) 30-60 minutes prior to sleep or an identical capsule (Capsuline, In., Pompano Beach, FL) containing white flour. Fasting blood draws occurred on three mornings: prior to supplementation, after 42 days, and after 84 days. This study was approved by the Old Dominion University Institutional Review Board, and all subjects provided signed informed consent prior to participation.
2.1. Blood Chemistry Analyses
- Blood samples were collected in heparinized tubes, in duplicate, by a trained phlebotomist from the antecubital vein using standard venipuncture technique. Lipids, fasting glucose and hemoglobin A1c were immediately analyzed using enzymatic assays (Cholestech Corp., Hayward, CA and Siemens Healthcare Diagnostics, Tarrytown, NY, respectively). Thereafter, samples were frozen and stored prior to spectrophotometric analysis of malondialdehyde (Zeptometrix Corp., Buffalo, NY).
2.2. Statistics
- Significant differences were analyzed using a 2-way ANOVA with repeated measures on one factor (time), whereas one-tailed, paired t-tests were used to assess change from baseline between groups. Results are presented as mean ±SEM. Data analyses were performed with PASW 17.0 (SPSS, Chicago, IL). Results were considered to be statistically significant if p < 0.05.
3. Results
- Levels of plasma malondialdehyde (MDA) were significantly lower following supplementation for MEL (-6.25±2.10 nmol/ml) compared to PLA (0.72±3.30, p=0.028) (Figure 1). This equates to a 55% reduction in MDA following melatonin supplementation.
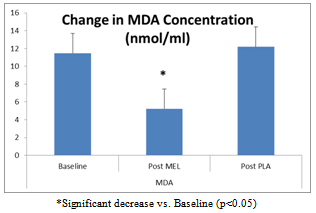 | Figure 1. Change in MDA Concentration Following 6 weeks of MEL or PLA |
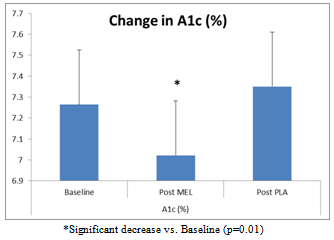 | Figure 2. Change in A1c % following 6 weeks of MEL and PLA |
|
4. Conclusions
- Similar to animal and human in-vitro data, this investigation demonstrated an ameliorative effect of melatonin supplementation on malondialdehyde, a common marker of oxidative stress. It also demonstrated that a single daily dose of 10 mg of melatonin significantly decreased overall glycaemia (i.e., A1c values) in individuals with T2D, thereby adding to the limited amount of in-vivo evidence demonstrating a potential role of melatonin in improving glycemic control in this population. Hyperglycemia present in T2D is a contributing factor to both a proinflammatory state and oxidative stress. T2D is also characterized by a reduction in antioxidative capacity[6]. It is widely accepted that oxidative stress plays a significant role in the pathogenesis of diabetes complications[1, 2]. While there is a strong theoretical background advocating the use of conventional antioxidant therapy to improve antioxidative status and oxidative stress, studies using supplemental vitamins C, E and beta-carotene have yielded disappointing results[30]. Given the lackluster performance of conventional antioxidants in addressing health-related outcomes induced by oxidative stress, it has been suggested that a new antioxidant approach to oxidative stress should focus on increasing intracellular antioxidant defenses and controlling free radical formation at its source[1]. The “holy grail” of antioxidants would be capable of directly scavenging free radicals, but more importantly, it would also be capable of: a) stimulating antioxidative enzymes, b) directly promoting glutathione synthesis, and c) reducing production of free radicals in the mitochondria. Human in-vitro studies have shown that melatonin reduces oxidative stress and up-regulates endogenous antioxidative defense[31, 32, 33]. Furthermore, melatonin has a demonstrated ability to neutralize oxidative stress in humans across a broad spectrum of conditions including malaria, post-surgery, new-born asphyxia and Alzheimer’s disease but, more importantly, in vascular disease and atherosclerosis as well[34].Melatonin differs from conventional antioxidants by serving both as a direct scavenger of ROS as well as a potent stimulator of endogenous antioxidant enzymes[4] via upregulation of gene expression for glutathione peroxidase, glutathione reductase, catalase, and superoxide dismutase[31, 35]. Importantly, it also affects the production of free radicals in the mitochondria[36], along with alleviating inflammation, possibly by inhibiting NF-κB, a key protein involved in inflammation[37]. This has potentially profound implications as the “unifying hypothesis” proposed by Brownlee[2] suggests that hyperglycemia-inducedoverproduction of the free radical superoxide, which occurs as a result of inefficiency in the electron transport chain, is the primary mediator of vascular damage in T2D. Therefore, any substance which enhances the efficiency ofmitochondrial respiration (thereby decreasing the production of superoxide at the source) may play a significant role in the reduction of complications that result from T2D.Interestingly, reductions in plasma melatonin are independently associated with T2D[14]. A recent large-scale investigation, a case-control study nested within the The Nurses’ Health Study, adds corroborating evidencedemonstrating a greater risk of development of T2D associated with decreased nocturnal melatonin secretion[38]. Consequently, this reduction in circulating melatonin, which is associated with significantly higher levels of oxidative stress and reduced antioxidant activity[6], is a likely result of an increased consumption of melatonin due to hyperglycemia precipitating increased levels of oxidative stress[39]. Relatively few studies have investigated the effect of exogenous melatonin on oxidant status and/or markers of oxidative stress in vivo in humans. Nevertheless, the few studies that have investigated this have demonstrated results similar to the present investigation. For example, a study involving young (35.9±2.3 yr) and elderly (79.4±.0 yr) healthy, normoglycemic populations found significant increases in superoxide dismutase and glutathione reductase, markers of antioxidative status, in healthy young and elderly subjects and a significant decrease in malondialdehyde, a marker of lipid peroxidation, in young (10%) and elderly (20%) subjects following 30 days of melatoninsupplementation at 5 mg/day[40]. Moreover, there was greater than a two-fold increase in serum melatonin levels for both youth and elderly population’s post - supplementation. The sole prior study that has investigated the effect of melatonin on oxidative stress in vivo in T2D was conducted in elderly patients[6]. Similar to the present investigation, a daily dose of melatonin (5 mg/day) for 30 days resulted in a substantial reduction in malondialdehyde (-20%), as well as an increase in superoxide dismutase (+16%), an important intracellular antioxidant defense, and serum melatonin increased by 70%.While there is an abundance of research in animal models demonstrating melatonin plays a significant role in glucose homeostasis[41, 42], there is a paucity of clinical trials investigating this effect in humans. Nevertheless, the limited human research has yielded intriguing results. For example, Hussain et al.[19] found that 90 days of supplementation of 10 mg melatonin, in combination with zinc (50 mg/day) significantly decreased fasting plasma glucose (-23%) and A1c (-29%) in individuals with T2D.While it is firmly established that oxidative stress is a significant contributing factor to the pathogenesis of T2D current therapy remains primarily aimed at pharmacological intervention to decrease endogenous glucose production, enhance insulin secretion, or decrease insulin resistance. The current treatment strategy, however, is less than optimal. Diabetic complications are a direct result of hyperglycemia and its associated oxidative stress, but only 37% of patients with T2D maintain an optimal glucose level[43]. Strategies that target oxidative imbalance and proinflammation may have the potential to ameliorate, delay, and/or prevent T2D[44].There is a growing body of evidence indicating that melatonin may play a multi-faceted role in the treatment of T2D. However, a paucity of clinical trials has investigated the impact of melatonin supplementation on oxidative status and glycemic control in a diabetic state. By resetting the suprachiasmatic nucleus, improving sleep, restoring circadian rhythmicity and reducing oxidative stress, melatonin may prove to be a low-cost adjunctive therapy in the treatment and prevention of T2D and its complications. Given the prevalence and rapid growth of diabetes and the totality of evidence linking melatonin to reduced oxidative stress and increased antioxidant defenses, further investigation into the possible mechanisms and use of this unique antioxidant appear warranted.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML