-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2012; 1(5): 92-95
doi: 10.5923/j.diabetes.20120105.04
Effects of Glycaemic Status on Plasma Levels of Calcium, Chromium, Copper, Iron, Magnesium, Selenium and Zinc in Diabetic Rats
Ugwuja E. I.1, Ugwu N. C.1, Aloke C2, Idenyi JN3, Nwibo AN1, Ibiam UA4, Ezenkwa US1
1Department of Chemical Pathology, Faculty of Clinical Medicine, Ebonyi State University, P.M.B. 053, Abakaliki, Nigeria
2Department of Medical Biochemistry, Faculty of Basic Medical Sciences, Ebonyi State University, P.M.B. 053 Abakaliki, Nigeria
3Department of Biotechnology, Faculty of Biological Sciences, Ebonyi State University, P.M.B .053, Abakaliki, Nigeria
4Department of Biochemistry, Faculty of Biological Sciences, Ebonyi State University, P.M.B .053, Abakaliki, Nigeria
Correspondence to: Ugwuja E. I., Department of Chemical Pathology, Faculty of Clinical Medicine, Ebonyi State University, P.M.B. 053, Abakaliki, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
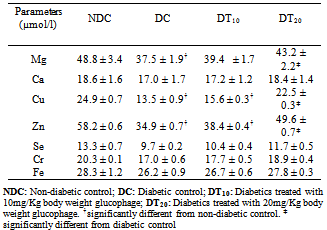
There is increasing evidence of the involvement of minerals in the pathogenesis of diabetes mellitus and its complications. In order to determine the effects of glycaemic status on the plasma levels of calcium, chromium, copper, iron, magnesium, selenium and zinc, 24 albino rats weighing 105-162 g grouped into 4 {non-diabetic control (NDC), diabetic control (DC), and diabetic treated with 10mg/Kg body weight (DT10) and 20mg/Kg body weight (DT20) of glucophage respectively}, were investigated. In addition to fasting plasma glucose, plasma levels of elements were determined by atomic absorption spectrophotometer. Results show that diabetic rats had lower levels of the elements in comparison to their non-diabetic counterparts but only magnesium {37.5 (1.9) vs. 48.8 (3.4); p = 0.033)}, copper {13.5 (0.9) vs. 24.9 (0.7); p = 0.032)} and zinc {34.9 (0.7) vs. 58.2 (0.6); p = 0.013)} were statistically significant. Again, higher levels of the elements were observed in diabetic treated rats when compared to the diabetic control but only copper {22.5 (0.3) vs. 13.5 (0.9), p = 0.043)} and zinc {49.6 (0.7) vs. 34.9 (0.7), p = 0.028)} were found to be significant, at higher dosage of the antihyperglycaemic agent. Plasma glucose was negatively correlated with copper (r =-0.273; p = 0.017), magnesium (r = -0.212; p = 0.024 and zinc (r = -0.245; p = 0.019), with no significant relationship observed among the elements. We conclude that hyperglycaemia of diabetes alters plasma mineral levels with plasma copper, magnesium and zinc being more responsive to alterations in glycaemic status than calcium, chromium, iron and selenium.
Keywords: Hyperglycaemia, Mineral Metabolism, Diabetic Complications, Antihyperglycaemic Agent
Cite this paper: Ugwuja E. I., Ugwu N. C., Aloke C, Idenyi JN, Nwibo AN, Ibiam UA, Ezenkwa US, "Effects of Glycaemic Status on Plasma Levels of Calcium, Chromium, Copper, Iron, Magnesium, Selenium and Zinc in Diabetic Rats", International Journal of Diabetes Research, Vol. 1 No. 5, 2012, pp. 92-95. doi: 10.5923/j.diabetes.20120105.04.
Article Outline
1. Introduction
- Diabetes mellitus, a metabolic syndrome characterized by hyperglycaemia and glycosuria is caused by absolute or relative lack of insulin, insulin resistance or both. It is estimated that diabetes affects about 170 million people world-wide[1]. Trace elements have been recognized as essential for optimal cellular functions, where they serve a variety of catalytic, structural and regulatory functions and in which they interact with macromolecules such as enzymes, pro-hormones, pre-secretory granules and biological membranes[2]. Although association of trace elements with health and disease has been established[3], the relationship between trace element metabolism and the aetiology and complications of diabetes mellitus is still a subject of intensive debate. For instance, while some studies[3-6] have documented lower plasma levels of zinc in diabetic subjects, others[2, 7, 8] reported no change in zinc levels. Similarly, reports on other trace elements in diabetes mellitus can best be described as inconsistent[2-8]. Although diabetes mellitus has been linked to perturbations of mineral metabolism, it is not clear whether it is diabetes and hyperglycaemia that affect mineral metabolism or if it is alterations in mineral homeostasis that influences carbohydrate metabolism[6, 9, 10. This study is therefore design to evaluate the effect of glycaemic status on the plasma levels of calcium, chromium, copper, iron, magnesium, selenium and zinc in alloxan - induced diabetic rats.
2. Materials and Methods
- Male Wister albino rats (n = 24), weighing 105-162 g purchased from animal house of the Department of Pharmacy, University of Nigeria, Nsukka were randomly assigned into four (4) groups (I-IV) of six (6) rats per group. They were kept in standard cages at 25℃ and 12 h light/dark condition in the animal room of the Department of Biochemistry, Ebonyi State University, Abakaliki. The animals were fed on commercial rats’ feeds and were given water ad libitum for a period of two week to allow them acclimatize. All the rats received human care in accordance with the National Institute of Health guidelines for the care and use of laboratory animals[11].Group I rats acted as non-diabetic control (NDC) and were maintained on feed and water. Rats in groups II-IV were made diabetic by intraperitoneal injection of 200 mg/Kg body weight of alloxan dissolved in distilled water. Fasting blood glucose levels were measured after three days of alloxan injection with a glucometer (ACCUTREND GC (Boerhinger, Mannheim, Germany), using blood from the tail tips and diabetes mellitus was confirmed by elevated blood glucose>7.8 mmol/l. Group II rats were thereafter maintained on feed and water and acted as diabetic control (DC). For the rats in group III (DT10) and IV (DT20) they were in addition to normal feed and water administered oral antihyperglycaemic agent-Metformin (glucophage) at a dose of 10mg/Kg body weight and 20mg/Kg body weight daily, respectively.The dosage of Metformin used in this experiment was arrived at based on the normal dosage used in human (1000-1500mg/day). For a 70Kg man, it means1000-1500mg/70Kg, which amounts to 14.3-21.4mg/Kg body weight. Thus we approximated the dose to 10-20mg/ Kg body weight. The experiment lasted for 28 days after which the rats were anaesthetized in a chloroform saturated chamber and the fasting blood samples were collected by cardiac puncture. The blood samples were dispensed into fluoride oxalate and heparinised bottles for the estimation of plasma glucose and mineral elements respectively. The blood was spun at 2000g for 5 minutes and plasma separated into previously chemically cleaned screw cap bottles. While plasma glucose was determined immediately, plasma for mineral element analyses were stored frozen (-8℃) before analyses. Plasma glucose was determined by glucose oxidase method[12] while plasma mineral elements were determined by atomic absorption spectrophotometer (AAS). Data were analysed for mean and standard deviation. Comparison of parameters among groups was done by one-way analysis of variance (ANOVA) and p value less than 0.05 was considered as statistically significant.
3. Results
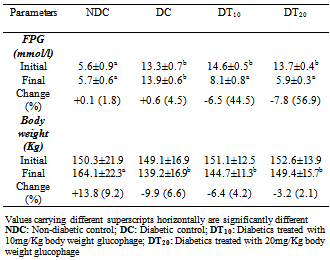
- The initial fasting plasma glucose levels of the diabetic rats irrespective of treatment were significantly higher when compared to the level in non-diabetic rats (Table 1). However, fasting plasma glucose levels were significantly lower in the treated rats in comparison to their untreated counterparts. The observed glucose lowering effect of glucophage was dose dependent, with a dose of 20mg/Kg body reducing plasma glucose by 56.9% against the 44.5% observed with a dose of 10mg/Kg body weight.
|
|
4. Discussion
- This study has showed that hyperglycaemia of diabetes generally alters plasma mineral element levels, but only magnesium, copper and zinc levels were significantly affected. Also weight loss in diabetes mellitus can be reduced by treatment with glucophage even as plasma glucose is reduced. Few studies were encountered on the influence of diabetic hyperglycaemia on plasma trace element levels and these studies assessed mainly tissue levels of trace elements and were old[13, 14]. For instance, in the study of the influence of chronic diabetes on tissue and blood cell status of Zn, Cu and Cr in rats, Razi and Havivi[13] reported elevated contents of Zn and Cu in the liver, femur, erythrocytes and lymphocytes of diabetic rats with increased urinary loss of the elements.Similarly, both Cu and Zn have been found to accumulate in the liver and kidneys of streptozotocin treated rats one week after injection and increased thereafter, attaining two and five fold higher, respectively by four weeks[14]. Significantly lower plasma Cu, Mg and Zn in diabetic rats in comparison to non-diabetic rats observed in the present study corroborated the finding of Hussain et al.,[3] where Zn and Mg levels were reported to be lower in diabetics in comparison to non-diabetics and that of Anjum et al.[4] in which significantly lower level of Zn was reported in diabetic subjects in comparison to their non-diabetic counterparts. It however contrasted lack of effects on plasma Zn, Cu and Cr reported by Babalola et al.[7] and on Zn[2] and Mg[2, 5] or on zinc[15], as well as higher plasma copper in diabetics reported by some authors[2, 4, 6]. Previously, significantly reduced mean levels of Zn, Mn, and Cr in blood and scalp-hair samples have been reported in diabetic patients when compared to control, with urinary excretion of the elements also significantly higher in diabetic subjects[13]. Also, low serum levels of Zn, Cr and Mg had been reported in diabetics compared to control subjects which the authors suggested may be due to the poor glycaemic control[17].Although, the cause of reduced levels of Cu, Mg and Zn observed in the present study is not obvious, it could be partly attributed to increased urinary excretion of the elements as a result of osmotic diuresis. It has been earlier reported that diabetes and poor glycaemic control alter the metabolism of zinc and magnesium by increasing their urinary excretion and lowering serum levels[18]. Alternatively, decreased intestinal absorption of the elements may also be a factor[19], although evidence supporting this proposal is still weak[18]. It has been speculated that hyperglycaemia may interfere with the active transport of zinc back into the renal tubular cells[19]. Alsodiabetic-associated glycosuria has been reported to impair renal tubular absorption of magnesium from the glomerular filtrate [20].The significantly higher plasma levels of Cu and Zn in diabetic rats treated with 20mg/Kg body weight of glucophage (DT20) in comparison to diabetic control (DC) observed in the present clearly shows that glycaemic control in diabetes mellitus have effect on plasma macro- and trace element levels. As blood glucose is brought down from 13.7 (0.4) to 8.1 (0.3), plasma Cu and Zn levels were increased from 13.5 (0.9) and 34.9 (0.7) µmol/l, respectively to 22.5 (0.3) and 49.6 (0.7) µmol/l, respectively. This observation is consistent with that of earlier study[18]. It is also in agreement with the study of Paolisso[21] who observed that glycaemic control in patients with type 2 diabetes may not correct low Mg concentration. Although the mechanism underlying these observations is not obvious, reduced urinary excretion of these elements with good glycaemic control may be a possibility as increased urinary excretions of trace elements in poor glycaemic control have been attributed to hyperglycaemia, glucosuria and osmotic diuresis[22].The significant inverse correlation between fasting plasma glucose and plasma Cu, Mg and Zn, respectively, observed in this study is in contrast with the finding of Akhuemokhan et al.[15] where serum concentration of Zn was neither correlated with fasting blood glucose nor glycated haemoglobin.Also there were no relationships among the trace elements. This is in contrast to a positive correlation between Cu and Zn in type 1 diabetes mellitus[8], negative correlation between Cu and Zn in diabetic patients[6] and between Zn and Mg reported among diabetics in Calabar, Nigeria[18]. The difference in the findings of these studies with the present study may be partly attributed to difference in subjects. The studies were either conducted in type 1 diabetes[8] or diabetics in general (type 1 and 2)[6, 18], unlike the present study which was done in type 1 diabetic modelAlthough in the present study, hyperglycaemia of diabetes seems to alter other trace elements, including Cr, Fe, Se and Ca, the effect was not significant, suggesting that their response to acute glycaemic alterations were not efficient. However, studies have associated these elements with either incidence of diabetes or pathogenesis of diabetic complications[15, 23, 24]. The proposed mechanism of action of trace elements in the pathophysiology of diabetes mellitus include improvements in insulin receptor / postreceptor signalling, leading to increased glucose transport by enhanced activity of the hormone-sensitive Glut-4 transporters[25], acting as a component or cofactor of enzymes involved in glucose metabolism[26], and acting as antioxidants, thus prevention the peroxidation of biomelucules[27, 28].
5. Conclusions
- This study reaffirms that diabetes mellitus alters trace element metabolism with plasma copper, magnesium and zinc being more responsive to alterations in glycaemic status than calcium, chromium, iron and selenium. However, further study is desired to evaluate the effect of short-and long-term effects of diabetic hyperglycaemia on plasma levels of mineral elements.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge the technical staff of the Departments of Chemical Pathology, Medical Biochemistry and Biotechnology for logistic support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML