-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2012; 1(4): 58-67
doi: 10.5923/j.diabetes.20120104.03
Ethanolic Leaf Extract of Croton Zambesicus (MÜll. Arg.) Improves Gastric Emptying Capacity and Gastric Mucosa Integrity in Streptozotocin-induced Diabetic Rats
David A. Ofusori , Omobola A. Komolafe , Olarinde S. Adewole
Department of Anatomy and Cell Biology, Faculty of Basic Medical Sciences, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria
Correspondence to: David A. Ofusori , Department of Anatomy and Cell Biology, Faculty of Basic Medical Sciences, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The aim of this study was to examine the effect of C. zambesicus leaf extract on the gastric emptying capacity, histomorphometry, nitric oxide (NO) and prostaglandin E2 (PGE2) levels in experimentally induced diabetic wistar rats. At the end of the experimental period, the animals were sacrificed and the stomach excised for gastric emptying analysis. Tissues were later processed and stained for histomorphometric analysis. Serum was obtained for NO and PGE2 analysis using respective research kits. The results showed that the percentage gastric emptying capacity were significantly increased (P<0.05) in group pretreated with C. zambesicus (78.40 ± 2.99%) and group treated with C. zambesicus for four weeks (72.80 ± 5.82%) when compared with the untreated diabetic group (39.20 ± 6.15%). NO and PGE2 were significantly reduced (p<0.05) in the untreated diabetic rats when compared with extract treated groups. Also, there was a significant (p<0.05) improvement in the histomorphometry of the extract treated groups as compared with the untreated diabetic group. In conclusion, this study had shown that C. zambesicus extract has a positive influence on gastric emptying capacity and gastric mucosa integrity in diabetic rats.
Keywords: Diabetes, Croton Zambesicus, Gastric Emptying, Nitric Oxide, Prostaglandin E2
Cite this paper: David A. Ofusori , Omobola A. Komolafe , Olarinde S. Adewole , "Ethanolic Leaf Extract of Croton Zambesicus (MÜll. Arg.) Improves Gastric Emptying Capacity and Gastric Mucosa Integrity in Streptozotocin-induced Diabetic Rats", International Journal of Diabetes Research, Vol. 1 No. 4, 2012, pp. 58-67. doi: 10.5923/j.diabetes.20120104.03.
Article Outline
1. Introduction
- Gastrointestinal (GI) disorders are common among all people, including those affected by diabetes[1]. At some point in any patient's life, there is always a chance that he or she will develop a GI tract problem[1].As many as 75% of patients visiting diabetes clinics will report significant GI symptoms[2]. The entire GI tract can be affected by diabetes from the oral cavity and esophagus to the large bowel and anorectal region[2]. Thus, the symptom complex that may be experienced can vary widely. Common complaints may include dysphagia, early satiety, reflux, constipation, abdominal pain, nausea, vomiting, and diarrhea[1]. Many patients go undiagnosed and under-treated because the GI tract has not been traditionally associated with diabetes and its complications[1, 2].Both acute and chronic hyperglycemia can lead to specific GI complications[1]. Diabetes is a systemic disease that may affect many organ systems, and the GI tract is no exception. As with other complications of diabetes, the duration of the disorder and poor glycemic control seem to be associated with more severe GI problems. Patients with a history of retinopathy, nephropathy, or neuropathy should be presumed to have GI abnormalities until proven otherwise, and this is best determined by asking a few simple questions[1].Many GI complications of diabetes seem to be related to dysfunction of the neurons supplying the enteric nervous system[1]. Just as the nerves in the feet may be affected in peripheral neuropathy, involvement of the intestinal nerves may lead to enteric neuropathy. This is a type of autonomic or "involuntary" neuropathy and may lead to abnormalities in intestinal motility, sensation, secretion, and absorption. Different nerve fibers can either stimulate or inhibit intestinal motility and function, and damage to these nerves can lead to a slowing or acceleration of intestinal function, giving rise to a variable symptom complex[1].Several different treatments may provide benefit in the management of diabetic gastroparesis. Consumption of frequent small meals may provide some symptomatic relief[3]. Avoidance of high-fat and high-fiber foods may be beneficial as well. It is common to recommend a liquid diet during an exacerbation of gastroparesis. As symptoms worsen, parenteral hydration and alimentation may be required. Nasogastric tube suction may also be used during severe episodes[3].C. zambesicus, a component of tiger bush is used in traditional medicine for the treatment of several ailments like diabetes, hypertension, urinary tract infections, malaria, gonorrhea arthritis, diarrhea and impotence[4, 5]. The therapeutic properties of the plant have been established by various workers. For instance, non-volatile extracts of the plant are known to possess: vesorelaxant, antidiabetic, antimalaria and antimicrobial properties[6-9]. Even though literature is scanty on the relationship between diabetes and C. zambesicus, the leaf extract of C. zambesicus has been documented to produce a significant(P<0.01) reduction in blood glucose level after a single dose of the extract(150mg/kg) and in prolonged treatment (for 7 days) [8]. The antidiabetic activity was comparable to that of a reference drug-chlorpropamide[8].Numerous medications have been shown to provide some benefit in the treatment of gastroparesis. Metclopropamide is a dopaminergic antagonist that enhances gastric emptying and has primary antiemetic properties. Unfortunately, it crosses the blood-brain barrier and causes frequent neurological side effects, such as sedation, tremor, confusion, dystonia, and, at times, tardive dyskinesia, which may or may not reverse after the drug is stopped[1, 3]. It is therefore expedient to search for a long lasting therapy with limited side effects in the management of gastrointestinal disorders associated with diabetes.This study was thus designed to evaluate the effect of C. zambesicus extract on gastric emptying capacity and gastric mucosa integrity in diabetic rats.
2. Materials and Methods
2.1. Animal Care
- Seventy adult male albino rats of the Wistar strain were procured and acclimatized for two weeks at the Animal Holdings of the College of Health Sciences, Obafemi Awolowo University, Ile-Ife, Nigeria before the commencement of the research work. Animals were fed with standard rat feed (Capfeeds, Ibadan) and given water liberally.All the animal experiments were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health, USA (NIH, 1985)[10].
2.2. Preparation of Plant Extract
- The leaves of C. zambesius Müll. Arg. (Euphorbiaceae) were collected from Dramatic art garden of the Obafemi Awolowo University, Nigeria, and taken to the herbarium of Botany Department, Obafemi Awolowo University for authentication and identification. Herbarium specimen number of the plant (UHI 16511) was obtained. The fresh leaves of the plant were air dried on a laboratory table for 30 days and reduced to power using squeezing and crushing machine (Daiki Rika Kogyo Co-ltd, Japan). The powder (400 g) was extracted with absolute ethanol (2.8L) for 72 hours. The extract was filtered using a filter paper. The filtrate obtained was concentrated in vacuo at 20 ℃ using a vacuum rotary evaporator (BÜchi Rotavapor R110, Schweiz). The extract obtained was partitioned between dichloromethane and water. The dichloromethane fraction was oven dried at 37℃. The fraction obtained (13.8 g, 3.5%) was dissolved in 10% tween 80 and administered orally at a dose of 200 mg/kg as the plant extract.
2.3. Experimental Design
- The animals were divided into seven groups as follows, with ten animals in each group. Group A: Control rats administered intraperitoneally with 0.1 M sodium citrate buffer (pH 4.5), Group B: Diabetic rats administered orally with 10% tween 80 for 4 weeks after the initial four weeks of diabetic induction, Group C: Diabetic rats but in which C. zambesicus leaf extract (200 mg/kg body weight/day/rat) in 10% tween 80 therapy started 2 weeks prior to induction and continued throughout the period the experiment lasted (8 weeks), Group D: Diabetic rats administered orally with C. zambesicus leaf extract (200 mg/kg body weight/day/rat) in 10% tween 80 for 2 weeks after the initial four weeks of diabetic induction (Withdrawal group), Group E: Diabetic rats administered orally with C. zambesicus leaf extract (200 mg/kg body weight/day/rat) in 10% tween 80 for 4 weeks after the initial four weeks of diabetic induction,Group F: Normal rats administered orally with C. zambesicus leaf extract (200 mg/kg body weight/day/rat) in 10% tween 80 for four weeks, Group G: Diabetic rats administered with glimepiride (2 mg/kg body weight/day/rat) in 10% teen 80 solution orally for four weeks[11] after the initial four weeks of diabetic induction.
2.4. Induction of Experimental Diabetes
- The animals in groups B, C, D, E and G were injected intraperitoneally with streptozotocin (Tocris Bioscience, UK, 65mg/kg body weight) dissolved in 0.1M sodium citrate buffer (pH 4.5). All the animals were kept and maintained under laboratory conditions of light, humidity and temperature. Before induction of diabetes, all the animals were fasted for 16-h, but still allowed free access to water throughout. At the end of the 16-h fasting period – taken as 0 time (i.e., 0 h) – blood glucose levels (initial glycemia) of the fasted normal (control), and other experimental rats were determined and recorded. These animals were stabilized for four weeks after which the leaf extract in 10% tween 80 was administered orally through gavages at a concentration of 200 mg/kg body weight/rat/day to groups D and E for another 2 and 4 weeks respectively. Meanwhile animals in group C were pretreated with C. zambesicus extract therapy 2 weeks prior to induction of diabetes.
2.5. Gastric Emptying Assessment
- Gastric emptying was studied in the control and experimental rats using solid markers. Glass beads (Adfolak Universal Venture Ltd, Nigeria), 1 mm in diameter, were used as nondigestible, nonabsorbable solid markers. Each rat was orally loaded with 50 glass beads[12] in physiological saline solution (3 ml/kg) through a gastric catheter (Adfolak Universal Venture Ltd, Nigeria). Two hours later, the rats were sacrificed. The 2-hour test period was selected because the leading bead after this period had traveled 80% of the small intestine and no beads could be found in the cecum[12].The stomach and the intestine were exposed. The glass beads in the stomach and intestine were counted. The gastric emptying was expressed as the ratio of the number of glass beads in the small intestine to that counted from the entire gastrointestinal tract[13, 14].
2.6. Serum Collection and Preparation
- Serum was obtained after centrifuging the blood samples collected via cardiac puncture for 5 minute at 5000 r.p.m. in a Benchtop Refrigerated centrifuge (Centurion Scientific centrifuge, R8000 series, UK). The serum obtained was used for the determination of NO and PGE2 using commercially available kit. (Randox, Northern Ireland).
2.7. Biochemical Assays in the Serum
2.7.1. Prostaglandin E2
- Prostaglandin E2 (PGE2) in the serum was determined using PGE2 EIA Monoclonal assay kit (Arbor Assays, USA) and based on the competitive binding technique in which PGE2 present in a sample competes with a fixed amount of horseradish peroxidase (HRP)-labeled PGE2 for sites on a mouse monoclonal antibody[15].
2.7.2. Nitric Oxide
- Nitric Oxide was determined using commercially available kits for the colometric determination of total nitrite (BioAssay Systems, USA) by reduction of nitrate to nitrite using improved Griess method[16].
2.8. Histomophometry
- Stomach tissues were processed for routine histological studies. The stained sections were subjected to morphometric analysis recommended by WorldHealth Organization W.H.O.[17] which included: dividing the eye piece occulometer into two 100 small divisions, the stage micrometer scale was made up to 1mm divided into 0.1mm divisions and each 0.1mm was divided into 0.01mm, the eye piece scale (occulometer) was inserted into the eye piece of the microscope by removing the superior lens thus placing the scale on the field stop, the stage micrometer was also placed on the stage of the microscope, the stage scale was focused by the low power objective lens (x4), the stage and the eye piece scales were adjusted until there was a parallel point between the two scales, the number of the eye piece divisions and its corresponding stage measurements was noted; (if 70 occulometer divisions equal to 14μm, all the objective lens were thus calibrated). Calibration was needed for each microscope use. The occulometer fixed into the Olympus Microscope was then focused through stained sections of the tissue to allow for the measurement of the gastric layers (mucosa, submucosa and muscularis externa)
2.9. Statistical Analysis
- Data were expressed as Mean ± Standard Error of Mean (S.E.M). The statistical significance was evaluated by one-way analysis of variance (ANOVA) using SPSS version 17.0 (SPSS, Cary, NC, USA) with Duncan's Multiple Range Test (DMRT) option. A value of p<0.05 was considered to indicate a significant difference between groups.
3. Results
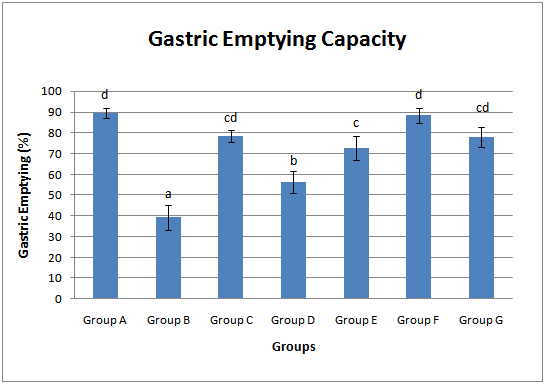
3.1. Gastric Emptying Capacity
- The gastric emptying capacities (%) in all animal groups were assessed. Untreated diabetic group (39.20 ± 6.15%) showed a significant (p<0.05) decrease in the gastric emptying capacity when compared with the control animals (89.60 ± 2.56%) (Fig. 1). With early commencement of extract administration two weeks prior to STZ induction, the gastric emptying capacity was increased to 78.40 ± 2.99%. Administration of the extract for four weeks after four weeks of diabetic stabilization (group E) also raised the gastric emptying capacity to 72.80 ± 5.82%. Withdrawal of the extract administration for two weeks significantly reduced the gastric emptying capacity by 37.05% and 22.53% when compared with the control and the group E (group which was administered with extract for four weeks after four weeks of diabetic stabilization). Group administered with glimepiride (group G) showed a significant increase (p<0.05) in gastric emptying capacity when compared with the untreated diabetic group (group B). The group administered with extract alone showed a non significant increase (p>0.05) in gastric emptying capacity vis-à-vis the control group (group A) (Fig. 1).
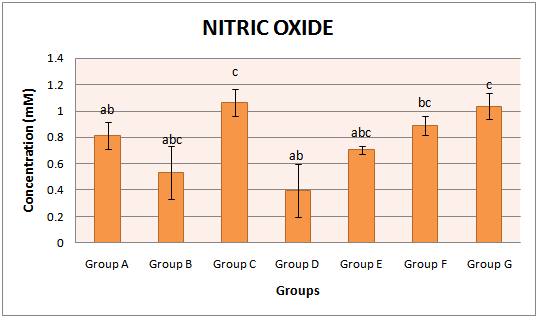
3.2. Nitric Oxide (NO)
- The activities of NO concentration in the serum of all animal groups were assayed. Untreated diabetic group (0.54 ±0.23 Mm) showed a significant (p<0.05) decrease in the activity of NO when compared with the control animals (0.81±0.12 Mm). With early commencement of extract administration two weeks prior to STZ induction, the activity of NO was increased to 1.06 ±0.10 Mm. Administration of the extract for four weeks after four weeks of diabetic stabilization (group E) also raised the activities of NO to 0.70 ±0.03 Mm. Withdrawal of the extract administration for two weeks significantly reduced the activities of NO by 50.62% and 42.86% when compared with the control and the group E respectively. Group administered with glimepiride (group G) showed a significant (p<0.05) increase in the NO concentration when compared with the group which was administered with extract for four weeks after four weeks of diabetic stabilization (group E). The group administered with extract alone also showed a significant (p<0.05) increment in the activities of NO vis-à-vis untreated diabetic group (group B) (Fig. 2).
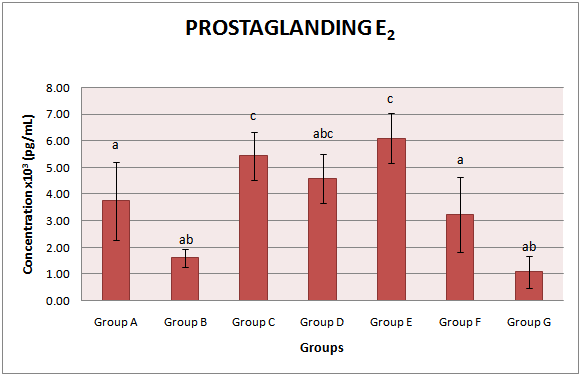
3.3. Prostaglandin E2 (PGE2)
- The activities of PGE2 concentration in the serum of all animal groups were measured. Untreated diabetic group (1.61 ± 1.34 x103 pg/mL) showed a significant (p<0.05) decrease in the concentration of PGE2 when compared with the control animals (3.75 ± 1.47 x103 pg/mL). With early commencement of extract administration two weeks prior to STZ induction, the activity of PGE2 was significantly (p<0.05) increased to 5.43 ± 1.91 x103 pg/mL. Administration of the extract for four weeks after four weeks of diabetic stabilization (group E) also raised the activities of PGE2 to 6.10 ± 0.93 x103 pg/mL. Withdrawal of the extract administration for two weeks significantly reduced the activities of PGE2 by 24.75% when compared with group E (group which was administered with extract for four weeks after four weeks of diabetic stabilization). Group administered with glimepiride showed a significant (p<0.05) reduction in the activities of PGE2 concentration when compared with the group which was administered with extract for four weeks after four weeks of diabetic stabilization (group E). The group administered with extract alone also showed a significant (p<0.05) increment in the activities of PGE2 vis-à-vis untreated diabetic group (group B) (Fig. 3).
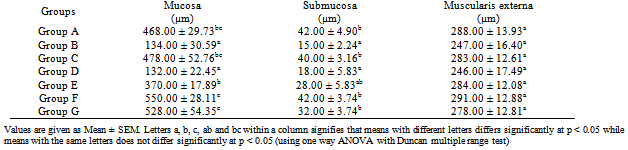
3.4. Histomorphometric Analysis
- The mucosa thickness was examined in all animal groups (Fig. 4-10). The mucosa thickness in the untreated diabetic group showed a significant reduction (p<0.05) when compared with the control animals. With early commencement of extract administration two weeks prior to STZ induction, there was significant (p<0.05) improvement in mucosa thickness when compared with the untreated diabetic group and control (Table 1). Administration of the extract for four weeks after four weeks of diabetic stabilization (group E) significantly (p<0.05) increased the mucosa thickness as compared with the untreated diabetic group and control. Withdrawal of the extract administration for two weeks drastically reduced the mucosa thickness in a significant manner (p<0.05) when compared with the control and group E (group which was administered with extract for four weeks after four weeks of diabetic stabilization) respectively. Group administered with glimepiride showed significant increase (p<0.05) in mucosa thickness when compared with the untreated diabetic group (Group B). The group administered with extract alone also showed a significant (p<0.05) increase in the mucosa thickness vis-à-vis control group (group A).The submucosa thickness in the untreated diabetic group showed a significant reduction (p<0.05) when compared with the control animals. With early commencement of extract administration two weeks prior to STZ induction, there was significant increase (p<0.05) in submucosa thickness when compared with the untreated diabetic group and control (Table 1). Administration of the extract for four weeks after four weeks of diabetic stabilization (group E) significantly (p<0.05) increased the submucosa thickness as compared with the untreated diabetic group and control. Withdrawal of the extract administration for two weeks drastically reduced the submucosa thickness in a significant manner (p<0.05) when compared with the control and group E (group which was administered with extract for four weeks after four weeks of diabetic stabilization) respectively. Group administered with glimepiride showed significant increase (p<0.05) in submucosa thickness when compared with the untreated diabetic group (Group B). The group administered with extract alone showed a fair correlation in the submucosa thickness vis-à-vis control group (group A).
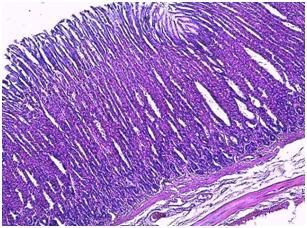 | Figure 4. Photomicrograph of the stomach of control group |
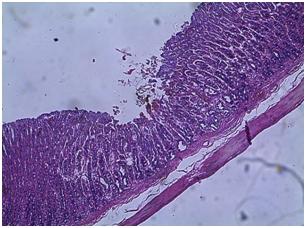 | Figure 5. Photomicrograph of the stomach of untreated diabetic group |
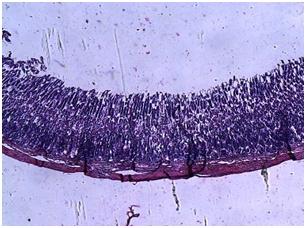 | Figure 6. Photomicrograph of the stomach of animals pretreated with C. zambesicus leaf extract 2 weeks prior to STZ-induction |
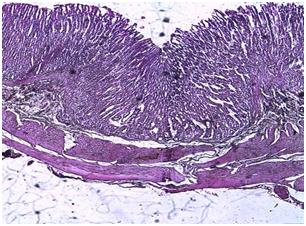 | Figure 7. Photomicrograph of the stomach of animals administered orally with C. zambesicus leaf extract for 2 weeks after STZ-induction (Withdrawal group) |
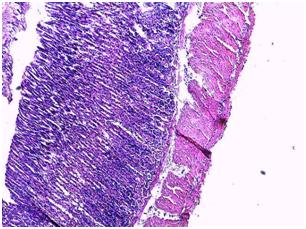 | Figure 8. Photomicrograph of the stomach of animals administered orally with C. zambesicus leaf extract for 4 weeks after STZ-induction |
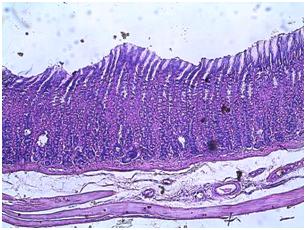 | Figure 9. Photomicrograph of the stomach of normal animals administered orally with C. zambesicus leaf extract for 4 weeks |
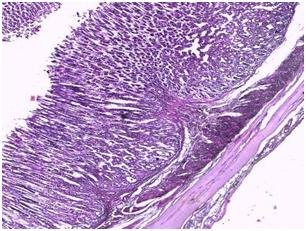 | Figure 10. Photomicrograph of the stomach of animals administered orally with glimepiride for 4 weeks after STZ-induction |
|
4. Discussion
- Relationships between diabetes and stomach have been of importance considering the vital role of the stomach in the body. Gastroparesis is one of diabetic gastrointestinal motor disturbances, and occur in approximately 50% of diabetic patients[18]. The pathogenesis of diabetic gastroparesis however remains poorly understood. Acute changes in blood glucose level affect gastric emptying in healthy men[18]. The results of the influence of diabetic hyperglycemia on solid gastric emptying in rats suggested that acute hyperglycemia was an important mechanism for the delay of solid gastric emptying in diabetic rats[18].Wang et al.,[12] and Wright et al.,[19] reported that liquid and solid meals emptied from the stomach at different rates. Solid markers of size 1mm in diameter were employed in this investigation. Other researcher such as Wang et al.,[12] and Luck et al.,[20] has also demonstrated gastric emptying capacity adopting the use of solid markers of 1mm in diameter. Two hours after loading the gastric beads in the animals, the gastric emptying capacity of the diabetic group was observed to be significantly reduced when compared with the control group. Hyperglycemia has been known to have a negative influence on gastrointestinal transit and duodenal-cecal transit[12, 21-24]. Diabetes is a leading cause of gastroparesis, accounting for about one-third of cases[12, 21]. Gastroparesis caused by damage to the vagus nerve is most often implicated in type 1 diabetes and type 2 diabetes[12, 21]. Damage to the vagus nerve keeps the muscles of the stomach and intestine from functioning properly. In gastroparesis, food stays in the stomach without usual peristatic activities to empty its content[25]. Pretreatment of animals with C. zambesicus two weeks before STZ induction and treatment of diabetic animals with C. zambesicus for four weeks significantly increased the gastric emptying capacity in the animal groups vis-à-vis the untreated diabetic group. This observation is consistent with the findings of Wang et al.,[12] and El-Lakany et al.,[26]. Following the withdrawal of the extract treatment, the gastric emptying capacity was reduced by 28.06% which affirmed the relevance of C. zambesicus in gastrointestinal transit of diabetic animals. More studies are expected to be carried out in this area to ascertain the mechanism by which C. zambesicus significantly increased the gastric emptying capacity.NO according to Taye and Saad[27], plays a critical role in modulating several component of gastric mucosa defence which include gastric mucosa blood flow, neutrophil adhension and mucus secretion thus affording gastric protection. Other investigators[28, 29] revealed that endogenous NO released from vascular endothelium, sensory nerves or gastric epithelium cooperates with endogenous prostaglandins in the maintenance of gastric mucosa integrity. Also, prostaglandins has been known to function in the protection of the stomach from injury by stimulating the secretion of bicarbonate and mucus, maintaining mucosa blood flow and regulating mucosa turn over and repair[30-33]. In this study, untreated diabetic group produced a significant reduction in NO and PGE2 levels. The reduction in NO may be connected with the production of superoxide anion radicals in the diabetic animals. Glucose oxidation which characterized diabetes leads to the production enediol radical anion that is converted into reactive ketoaldehydes and to superoxide anion radicals[34-36]. Superoxide anion radicals so generated has been reported to have high affinity for nitric oxide to form reactive peroxynitrite radicals thus depleting NO from the circulation[35, 36]. De La Cruz et al.,[37] also proposed that sustained high blood glucose levels in diabetes leads to oxidative stress and a decrease in NO production, thus contributing to the vascular dysfunction observed in DM. This reduction in NO may have resulted in concomitant reduction in mucosa blood flow by vasodilation and secretion of bicarbonate and mucus. Pretreatment of the diabetic animals with C. zambesicus and treatment of diabetic animals with C. zambesicus for four weeks however increased the synthesis of NO and PGE2 thus reducing the susceptibility of the stomach mucosa to injury and regeneration of the gastric lesion brought about by the STZ induction. Mohan and Das[38] reported a decrease in nitric oxide levels in the plasma of diabetic rats, an effect that was abrogated by prior and simultaneous administration of L-arginine, a precursor of nitric oxide. Recent studies have shown that insulin sensitizing agent increase NO production and enhance ulcer healing an effect which was abolished by pretreatment of L-NNA, an NO synthase inhibitor[39]. The increase in NO levels by C. zambesicus could be by activation of NO synthase by phosphorylation and increase NO bioavailability. This is consistent with the works of Taye and Saad[27] and Boyle et al.,[40]. However, increased production of NO may be one of the potential targets for the gastroptotective effect of C. zambesicus. A marked reduction in the concentration of PGE2 is associated with development of gastric ulcer[27]. Thus, its increament by C. zambesicus may possibly be by means of the stimulating effect of C. zambesicus on NO production. Taye and Saad[27] reported that NO increased PGE2 invivo through cGMP-independent mechanism and it was assumed that NO might regulate the release and/or the synthesis of PGE2 in the stomach after damage.A significant decrease observed in the thickness of the mucosa of the untreated diabetic group may be related to the decline in the cellular density which may be connected with the generation of oxidative stress in diabetic animals[34]. Prereatment of animals with C. zambesicus two weeks prior to STZ induction (Group C) and treatment of diabetic animals with C. zambesicus for four weeks (Group E), presented a significant (p<0.05) increase in the thickness of the mucosa. Following the withdrawal of extract treatment (Group D), the mucosa thickness was reduced. This demonstrated the ameliorative potential of C. zambesicus which further affirms the protective and regenerative efficacy of C. zambesicus[41]. Significant decrease observed in the thickness of the submucosa of the untreated diabetic group may be related to the depletion of the submucosa of the connective tissue fibers (collagen and elastic)[42]. Pretreatment of animals with C. zambesicus two weeks prior to STZ induction (Group C) and treatment of diabetic animals with C. zambesicus for four weeks (group E), restored the thickness of the submucosa to near normal. Following the withdrawal of extract treatment (Group D), the submucosa thickness was reduced. This observation has made evident the functional role of C. zambesicus in the preservation of connective tissue fibers which further establishes the protective and regenerative efficacy of the C. zambesicus. Significant decrease observed in the thickness of the muscularis externa of the untreated diabetic group may be related to the depletion of the elastic tissue fibers. Elastic fibers present in the muscularis externa are responsible for the churning and peristaltic movement which characterized the gastrointestinal tract[43]. Pretreatment of animals with C. zambesicus two weeks prior to STZ induction (Group C) and treatment of diabetic animals with C. zambesicus for four weeks (Group E), restored the thickness of the muscularis externa to near normal. These observations justify the functional implication of C. zambesicus in improving the churning and peristaltic activities of the stomach in diabetic rats by means of a possible increase in the elastic tissue fibers.In conclusion, this study had shown that C. zambesicus extract has a positive influence on gastrointestinal transit and gastric mucosa defense in diabetic rats.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML