-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2012; 1(4): 52-57
doi: 10.5923/j.diabetes.20120104.02
Effect of Simvastatin on the Pharmacodynamic Activity of Repaglinide in Rats/Rabbits
Makula Chandra Sekhar 1, 2, Jaya Chandra Reddy P 3
1Research Scholar, Jawaharlal Nehru Technological University, Hyderabad, Andhra Pradesh, India
2Gurram Balanarasaiah Institute of Pharmacy, Ghatkesar, Hyderabad, R.R.Dist, Andhra Pradesh, India
3Principal, KrishnaTeja Pharmacy College, Tirupathi, Andhra Pradesh, India
Correspondence to: Makula Chandra Sekhar , Research Scholar, Jawaharlal Nehru Technological University, Hyderabad, Andhra Pradesh, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Dyslipidemia is common in patients with type 2 diabetes. Statins are used as the first choice in treatment of diabetic dyslipidemia. Simvastatin, an HMG-CoA reductase inhibitor, is widely used in the treatment of hypercholesterolemia. Repaglinide is a short-acting, oral, insulin secretagogue that is used in the treatment of type 2 diabetes mellitus. Both the category of drugs undergo extensive metabolism with cytochrome P450 enzyme system. This may lead to drug-drug interaction problems with altered repaglinide activity which is unwanted. Simvastatin/repaglinide/simvastatin+repaglinide were administered orally to normal, diabetic rats, and to normal rabbits. Blood samples were collected at different time intervals and were analyzed for blood glucose by GOD–POD method using commercial glucose kits and repaglinide estimation in plasma by HPLC method. Diabetes was induced by alloxan 100 mg/kg body weight administered by I.P route. Simvastatin + repaglinide produced hypoglycemic activity in a dose dependant manner in normal and diabetic condition. In the presence of simvastatin, repaglinide blood glucose lowering activity was not increased significantly (P<0.05) compared with repaglinide matching control. The present study concludes co-administration of simvastatin was not improved repaglinide responses significantly in animal models.
Keywords: Repaglinide, Simvastatin, Diabetic Dyslipidaemia, Rats, Rabbits
Cite this paper: Makula Chandra Sekhar , Jaya Chandra Reddy P , "Effect of Simvastatin on the Pharmacodynamic Activity of Repaglinide in Rats/Rabbits", International Journal of Diabetes Research, Vol. 1 No. 4, 2012, pp. 52-57. doi: 10.5923/j.diabetes.20120104.02.
Article Outline
1. Introduction
- The incidence of cardiovascular disease (CVD) is more common in patients with type 2 diabetes than in the general population[1]. Statin therapy is recommended as the initial pharmacological treatment for lowering LDL-C levels in patients with type 2 diabetes who either have overt CVD or are over 40 years old and have increased CVD risk[2]. Statins are highly effective in reducing LDL and modestly effective in raising HDL. Triglyceride lowering is directly proportional to the baseline triglyceride level and to the LDL-lowering potency of the drug[3, 4].Repaglinide is a novel post prandial glucose regulator for the treatment of type 2 diabetes mellitus[5]. It reduces fasting glucose concentrations in patients with type 2 diabetes mellitus. It helps to control blood sugar by stimulating release of insulin from the pancreatic β-cell by closure of potassium ATP channels[6]. Compared to sulphonylureas, the meglitinides are thought to reduce the incidence of hypoglycaemic events because they promote insulin secretion only in the presence of hyperglycaemia, whereas sulphonylureas have been shown to increase insulin secretion at considerably low concentrations of blood glucose[7]. In addition, studies have shown repaglinide to potentially reduce the risk of diabetes associated complications like macro- and microvascular disease through the reduction of oxidative stress, a potent contributor of vascular dysfunction[8]. Simvastatin, an HMG-CoA reductase inhibitor, is widely used in the treatment of hypercholesterolemia. It has two separate metabolic pathways. The oxidative biotransformation of simvastatin is mediated primarily by CYP3A4[9].The study of mechanisms of drug interactions is of much value in diabetes mellitus therapy, because this is one such metabolic disorder that needs treatment for prolonged periods and maintenance of normal blood glucose level is very important in this condition, since both hyperglycaemia as well as hypoglycaemia is unwanted phenomenon[10]. Since there is every possibility for the combined use of repaglinide and simvastatin (statins) in diabetic dyslipidemia, the study is planned to investigate the effect of simvastatin on blood glucose and their effect on the activity of repaglinide in rats and rabbits to evaluate the safety and effectiveness of the combination with respect to blood glucose level.
2. Materials and Methods
- Repaglinide and simvastatin were obtained from Orchid Chemicals Ltd. (Chennai, India) and Aurobindo Pharma Ltd (Hyderabad, India), respectively, as gift samples. Alloxan monohydrate was purchased from LOBA Chemie (Mumbai, India). Indomethacin was purchased from Sigma-Aldrich (Bangalore, India). Glucose kits (Span diagnostics) were purchased from local pharmacy. All other reagents/chemicals used were of analytical grade. Albino rats of either sex of 7–8 weeks of age, weighing between 260 and 320 g and normal albino rabbits of either sex of 3 months of age, weighing between 1.8 and 2.1 kg were used in the study. They were procured from National Institute of Nutrition, Hyderabad, India. They were maintained under standard laboratory conditions at an ambient temperature of 25 ± 2°C and 50 ± 15% relative humidity with a 12 h light/12 h dark cycle. Animals were fed with a commercial pellet diet (Rayan’s Biotechnologies Pvt Ltd., Hyderabad, India) and water ad libitum. They were fasted for 18 h prior to the experiment and during the experiment they were withdrawn from food and water. The animal experimental procedures and protocols were carried out according to the guidelines provided by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
2.1. Study Design
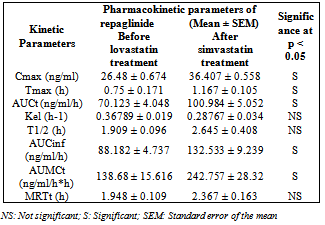
- In clinical practice, both simvastatin and repaglinide were administered orally in the treatment of diabetic dyslipidemia. Hence, human oral therapeutic doses of the respective drugs were extrapolated to rat/rabbit based on body surface area[11]. The dose of repaglinide for rat experiments was selected as 0.18 mg/kg bd.wt. based on the influence of dose–effect relationship of repaglinide on blood glucose in normal rats (i.e., for 200 gm rat dose = human dose X 0.018)[11]. The dose of repaglinide for rabbit experiments was selected as 0.14 mg/1.5 kg bd.wt. based on the influence of dose–effect relationship of repaglinide on blood glucose in normal rabbits (i.e., for 1.5 kg rabbit dose = human dose X 0.07).[11] Simvastatin and repaglinide were suspended in 0.5% CMC and in 1.5% methylcellulose for oral administration[12, 13]. The study consists of three stages:Stage-1 Study in normal rats (pharmacodynamic study).Stage-2 Study in diabetic rats (pharmacodynamic study).Stage-3 Study in normal rabbits (pharmacodynamic and Pharmacokinetic study).Study in normal/diabetic rats (pharmacodynamic study)A group of six rats was administered with 0.18 mg/kg bd. wt of repaglinide, orally. The same group was administered with interacting drug (simvastatin 3.6 mg/kg bd. wt. orally) and the combination of interacting drug and repaglinide. One week washout period was maintained between treatments. The same treatment as described in the study in normal rats was performed with a group of six alloxan-induced diabetic rats. The diabetes was induced by the administration of alloxan monohydrate in two doses, i.e. 100 mg and 50 mg/kg bd. wt. intraperitoneally for two consecutive days[14]. Blood samples of each 0.5 ml volume were withdrawn from retro orbital plexus[15] of each rat at 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, and 6 h. These blood samples were analyzed for blood glucose by GOD/POD method[16] using commercial glucose kits.Study in normal rabbits (pharmacodynamic and Pharmacokinetic study)A group of six rabbits was administered with 0.14 mg/1.5 kg bd. wt of repaglinide, orally. The same group was administered with interacting drug (simvastatin 2.8 mg/1.5 kg bd. wt. orally) and the combination of simvastatin and repaglinide. One week washout period was maintained between treatments. One week washout period was maintained between treatments. Blood samples of each 2 ml volume were withdrawn from the marginal ear vein of each rabbit at 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, and 8 h. These blood samples were analyzed for blood glucose by GOD/POD method[16] and for plasma repaglinide by HPLC method, as described by Wahman LF and colleagues,[17] and were modified in the laboratories. A gradient High Pressure Liquid Chromatograph (Shimadzu Japan) equipped with C18 reversed-phase column was used. Indomethacin was used as internal standard and the mobile phase consisted of acetonitrile: ammonium acetate buffer, 10 mM (pH 4.0) in a ratio of 50:50. The mobile phase was eluted at a flow rate 1 ml/min at 30°C and the effluent was monitored at a wavelength of 244 nm. The ratio of peak area of repaglinide to that of internal standard was used for the quantification of repaglinide in plasma samples. The HPLC method was validated in terms of reproducibility, system suitability, recovery, accuracy, and precision and then applied for the estimation of repaglinide in rabbit plasma.The plasma concentration–time data were analyzed by non-compartmental analysis using the WinNonlin Software (Version 5.0.1 from Pharsight Corporation, USA). The Cmax was maximum measured plasma concentration over the time span specified and Tmax was time of the maximum measured plasma concentration. If the maximum value occurs at more than one time point, Tmax is defined as the first time point with this value. Area under the concentration– time curve (AUCt) was calculated from time zero to the last measurable concentration by the linear trapezoidal rule. The elimination rate constant (Kel) was determined by linear regression analysis of the log-linear part of the plasma drug concentration–time curve. A minimum of three data points were used to calculate the elimination rate constant (Kel). The terminal half-life was calculated by using the formula, T1/2 = ln2/Kel. Area under the concentration–time curve from time zero to infinity (AUCinf) was calculated by the sum of the AUCt plus the ratio of the last measurable plasma concentration (Ct) to the elimination rate constant Kel. The AUMCt was area under the moment curve from the time zero to the last measurable concentration. The mean residence time (MRTt) was calculated using the formula, MRTt = AUMCt/AUCt.
3. Data and Statistical Analysis
- Data were expressed as mean ± SEM. The significance was determined by applying Student's paired 't' test. Significance was established at a p value of less than 0.05.
4. Results
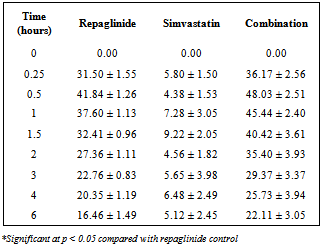
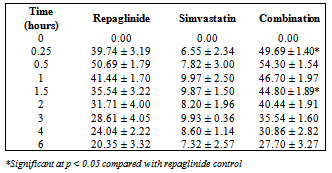
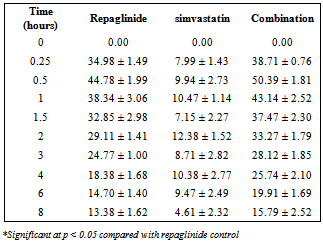
- Repaglinide produced rapid hypoglycemic activity with maximum reduction of 41.89 ± 1.46 and 44.53 ± 2.21% at 30 min in normal rats and rabbits, respectively, and produced antihyperglycemic activity with maximum percentage reduction of 48.00 ± 1.06% at 15 min in diabetic rats. The average percentage blood glucose reduction with repaglinide, simvastatin and their combination in normal rats, diabetic rats, and in normal rabbits is shown in the Tables 1, 2 and 3, respectively, and graphical representation is shown in Figs. 1, 2, and 3, respectively. Simvastatin alone was not produced significant effect on the blood glucose level of rats (normal and diabetic) and rabbits (Tables 1, 2, and 3). When administered in combination, simvastatin significantly increased the effect of repaglinide in diabetic rats (56.60 vs. 46.01%; P<0.05).
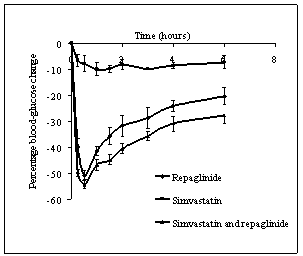 | Figure 1. Percentage blood-glucose reduction with repaglinide, simvastatin and their combination in normal rats (n = 6) |
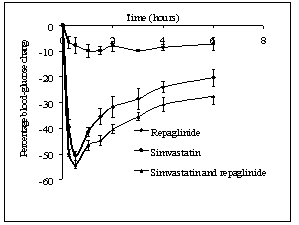 | Figure 2. Percentage blood-glucose reduction with repaglinide, simvastatin and their combination in diabetic rats (n = 6) |
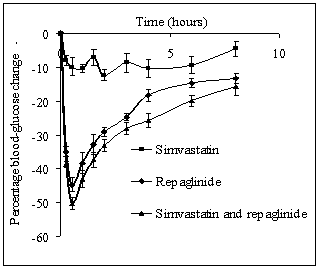 | Figure 3. Percentage blood-glucose reduction with repaglinide, simvastatin and their combination in normal rabbits (n = 6) |
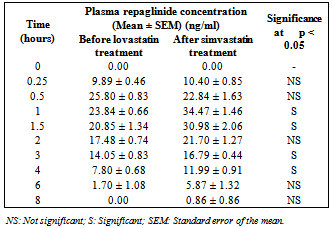
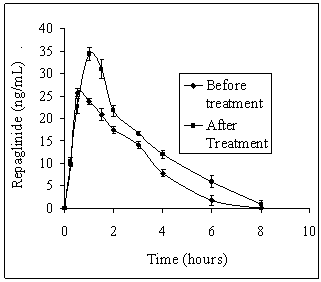 | Figure 4. Average plasma repaglinide concentrations before and after treatment with simvastatin in normal rabbits (n = 6) |
|
|
|
|
|
5. Discussion
- Our study revealed the no glucose lowering effect of simvastatin alone in diabetic condition, with respect to blood glucose levels. The activity of repaglinide was increased in the presence of simvastatin in diabetic rats where as in normal rats and in rabbits the effect was insignificant. Even though in normal rabbits, the plasma concentrations at 1, 1.5, 3 and 4 hours and pharmacokinetic parameters are increased significantly (P < 0.05) but average percentage glucose reduction is insignificant with combination treatment compared with repaglinide control treatment. Dyslipidaemia is common in patients with type 2 diabetes[18] and hence generally statins are co-administering along with oral antidiabetic drugs. Repaglinide is an oral insulin secretagogue that reduces postprandial glucose excursions by targeting postprandial insulin release[19]. Statin medications are the mainstay for treatment of patients with diabetes who have dyslipidemia[20]. Simvastatin has become one of the worlds most frequently prescribed drug for lipid lowering effect with good tolerability and large outcome trials proved, simvastatin provided substantial cardiovascular risk reduction[21]. Based on these factors the study was planned to investigate the effect of simvastatin on blood glucose and its effect on the activity of repaglinide in rats (normal and diabetic) and rabbits to evaluate the safety and effectiveness of the combination with respect to blood glucose level.Repaglinide is extensively metabolized by the hepatic cytochrome P450 enzyme system (CYP3A4 and CYP2C8), with less than 2% of an oral dose being excreted unchanged in humans[22, 23]. Simvastatin is primarily metabolized by CYP3A4 enzyme system[9]. Studies of by human recombinant P450 isoforms indicated that both CYP3A4 and CYP3A5 metabolized simvastatin efficiently and also simvastatin was an inhibitor of CYP3A enzyme system with a Ki value of ~10 µM. Further studies on the kinetics of simvastatin metabolism indicated that CYP3A4 possessed relatively higher affinity (3-9 fold) than CYP3A5[9]. So the increased pharmacokinetic activity of repaglinide in the presence of simvastatin may be due to competitive inhibition of CYP3A4 enzyme system.
6. Conclusions
- In conclusion, this study demonstrated that the interaction between simvastatin and repaglinide is insignificant and this will probably be a safe combination of drugs in humans too. Even though pharmacokinetic parameters of repaglinide increased significantly in the presence of simvastatin, the pharmacodynamic nature of repaglinide was not increased significantly. Further studies are needed to establish its long-term safety in animals and humans.
ACKNOWLEDGEMENTS
- We are thankful to Shekar Sunkoju (Biostatistian, Max Neeman International, New Delhi-110020) for assistance in statistical analysis. We are also thankful to Murali Krishna Matta (Senior Scientist, Bioanalysis, Synegene-BBRC, Bangalore) and Vijay Kumar mamidi (Scientist, Bioanalysis, Synegene Internatonal, Bangalore) for their kind support and guidance in bioanalytical method development and estimation of repaglinide in rabbits. The authors declare that they do not have any conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML